Diphosgene
Synonym(s):Diphosgene;di-Phosgene;TCF
- CAS NO.:503-38-8
- Empirical Formula: C2Cl4O2
- Molecular Weight: 197.83
- MDL number: MFCD00015553
- EINECS: 207-965-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
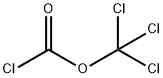
What is Diphosgene?
Description
Diphosgene (DP), trichloromethyl chloroformate, is a clear, colorless liquid with an odor similar to phosgene. It is noncombustible, a strong irritant to the eyes and tissues, and is toxic by inhalation and ingestion. DP has a boiling point of 127°C–128°C (263°F) and a vapor pressure of 4.2 at 68°F (20°C). The liquid density is 1.65, which is heavier than water, and a melting/freezing point of 314°F (157°C). Inhalation LC50 is 3600 mg/m3 for 10 min. Effects of exposure are quite similar to phosgene gas. Its molecular formula is ClCOOCCl3, and the structure and molecular formula are shown in Figure 8.38. The DOT lists diphosgene as a 6.1 poison liquid. The NFPA 704 designation for CG is estimated to be health 4, flammability 0, reactivity 1, and special 0. It has a four-digit UN identification number of 2972.
Description
Diphosgene is a compound that acts chemically like two equivalents of phosgene. This is because upon reacting diphosgene forms one equivalent of phosgene (toxic gas). Diphosgene, being a liquid, is more convenient to handle than phosgene. Triphosgene, which is a solid, is a related compound that chemically acts like three equivalents of phosgene.
Chemical properties
clear colorless liquid
The Uses of Diphosgene
In organic synthesis; as war gas.
The Uses of Diphosgene
Trichloromethyl chloroformate is used as a reagent in the synthesis of organic compounds. It serves as a source of phosgene used in some laboratory preparations. Also, it is used as a reactant for the synthesis of cyclic carbamimidates, N-alkenyl and cycloalkyl carbamates and prostate-specific membrane antigen-targeted anticancer prodrugs. In addition, it is involved in the preparation of an erythromycin A derivatives and antibody-drug conjugates. It is utilized in the conversion of amines, carboxylic acids, formamides in to isocyanates, acid chlorides and isocyanides respectively.
The Uses of Diphosgene
Reactant for preparation of:
- Cyclic carbamimidates using a monophosphine gold(i) catalyst
- N-Alkenyl and cycloalkyl carbamates as dual acting histamine H3 and H4 receptor ligands
- Prostate-specific membrane antigen-targeted anticancer prodrugs
- Potential west nile virus protease inhibitors
- Antibody-drug conjugates (ADCs)
- Erythromycin A derivatives
Definition
diphosgene: A colourless liquid,ClCO.O.CCl3, originally used in 1916by Germany in World War I as achemical warfare agent. It is nowused as a reagent in organic synthesis.See also carbonyl chloride.
General Description
Colorless liquid, odorless to fruity.
Air & Water Reactions
Decomposes slowly in water or moist air (or when inhaled) to form very corrosive hydrogen chloride gas (hydrochloric acid) and carbon monoxide.
Hazard
Toxic by inhalation and ingestion, strong irritant to tissue.
Fire Hazard
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Low toxicity by inhalation. A corrosive liquid. When heated to decomposition it emits toxic vapors of Cl-.
Properties of Diphosgene
| Melting point: | -57 °C |
| Boiling point: | 20 °C10 mm Hg(lit.) |
| Density | 1.64 |
| refractive index | n |
| Flash point: | >110°C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Soluble), Ethyl Acetate (Sparingly) |
| form | Liquid |
| appearance | Liquid |
| color | Clear colorless |
| Water Solubility | may decompose |
| Sensitive | Moisture Sensitive |
| Merck | 14,3334 |
| BRN | 970225 |
| Stability: | Hygroscopic, Moisture Sensitive |
| CAS DataBase Reference | 503-38-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Trichloromethyl chloroformate(503-38-8) |
| EPA Substance Registry System | Carbonochloridic acid, trichloromethyl ester (503-38-8) |
Safety information for Diphosgene
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Diphosgene
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 503-38-8 DIPHOSGENE 98%View Details
503-38-8 DIPHOSGENE 98%View Details
503-38-8 -
 TRICHLOROMETHYLCHLORO FORMATE CAS 503-38-8View Details
TRICHLOROMETHYLCHLORO FORMATE CAS 503-38-8View Details
503-38-8 -
 Diphosgene CAS 503-38-0View Details
Diphosgene CAS 503-38-0View Details
503-38-0 -
 DIPHOSGENE 98% CAS 503-38-8View Details
DIPHOSGENE 98% CAS 503-38-8View Details
503-38-8 -
 Trichloromethyl chloroformate CAS 503-38-8View Details
Trichloromethyl chloroformate CAS 503-38-8View Details
503-38-8 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0