Allyl chloroformate
- CAS NO.:2937-50-0
- Empirical Formula: C4H5ClO2
- Molecular Weight: 120.53
- MDL number: MFCD00000648
- EINECS: 220-916-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
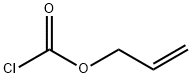
What is Allyl chloroformate?
Chemical properties
Colorless liquid
The Uses of Allyl chloroformate
Allyl Chloroformate is used in the synthesis of poly(ethylene oxide) hybrid systems for therapeutic applications and drug delivery. Also used in the synthesis of benzothiazole derivatives with potent anti-tumor properties.
General Description
A colorless liquid with a pungent odor. Flash point 88°F. Corrosive to metals and tissue. Very toxic by inhalation, ingestion and/or skin contact. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Water reaction produces heat and acidic HCl. In the presence of moist air, corrosive hydrogen chloride is produced.
Reactivity Profile
Acid halides, such as Allyl chloroformate, are water reactive; some are violently reactive. They are incompatible with strong oxidizing agents, alcohols, amines, alkali. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
Vapor irritates eyes and respiratory tract. Contact with liquid causes eye and skin irritation, and ingestion irritates mouth and stomach.
Chemical Reactivity
Reactivity with Water Reacts slowly generating hydrogen chloride; Reactivity with Common Materials: Corrosive metals; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water, rinse with sodium bicarbonate solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Poison by inhalation and ingestion. Corrosive. Dangerous when exposed to heat, open flame (or sparks), or powerful oxidizers. Can react with oxidizing materials. To fight fire, use alcohol foam, spray or mist, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also ALLYL COMPOUNDS and ESTERS.
Properties of Allyl chloroformate
| Melting point: | 106℃ |
| Boiling point: | 109 °C |
| Density | 1.134 g/mL at 20 °C(lit.) |
| vapor pressure | 3.67 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 88 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform |
| form | Liquid |
| color | Clear slightly yellow to light orange to reddish brown |
| Odor | Extremely irritating, causes tears; pungent. |
| BRN | 773915 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 2937-50-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Allyl chloroformate(2937-50-0) |
| EPA Substance Registry System | Carbonochloridic acid, 2-propen-1-yl ester (2937-50-0) |
Safety information for Allyl chloroformate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H301:Acute toxicity,oral H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Allyl chloroformate
Allyl chloroformate manufacturer
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Allyl Chloroformate 2937-50-0 98%View Details
Allyl Chloroformate 2937-50-0 98%View Details
2937-50-0 -
 2937-50-0 98%View Details
2937-50-0 98%View Details
2937-50-0 -
 2937-50-0 99%View Details
2937-50-0 99%View Details
2937-50-0 -
 Transparent Allyl Chloroformate CAS RN : 2937-50-0, LiquidView Details
Transparent Allyl Chloroformate CAS RN : 2937-50-0, LiquidView Details
2937-50-0 -
 Allyl ChloroformateView Details
Allyl ChloroformateView Details
2937-50-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
