Diphenylacetonitrile
Synonym(s):Benzhydryl cyanide, Diphenylmethyl cyanide;Diphenylacetonitrile
- CAS NO.:86-29-3
- Empirical Formula: C14H11N
- Molecular Weight: 193.25
- MDL number: MFCD00001862
- EINECS: 201-662-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-31 18:46:13
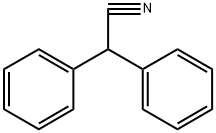
What is Diphenylacetonitrile?
Chemical properties
Diphenylacetonitrile is a white to creamy or faint yellow crystalline powder, Soluble in ethanol, ether. It is obtained by bromination and condensation of phenylacetonitrile. Diphenylacetonitrile is mainly used as an intermediate to manufacture API which deals in treatment of respiratory stimulant. It is used to manufacture APIs like stomach amine, Aminepentamide Sulphate, Diphenoxylate, Diphenylacetaldehyde, Doxapram, Loperamide, Methadone.
The Uses of Diphenylacetonitrile
Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceuticals.
Diphenylacetonitrile is used to synthesize isocyanate, which is further prepared into UV paint, PU paint, transparent elastomer and adhesive, etc. In addition, it is also used in polyamide and epoxy resin industries.
Preparation
Diphenylacetonitrile can be prepared in good yield by the dehydration of the amide of diphenyl acetic acid with phosphorous oxychloride. Diphenylacetonitrile undergoes anhydrous condensation with ethyl-4-bromo-butyrate. This is followed by its hydrolysis in alkaline medium that is used in the quantitative determination of 3-cyano-3,3-diphenylpropionic acid.
Preparation
In a 100 mL, nitrogen-flushed pear flask was added indium(III) bromide (2.0 mmol, 10 mol %) in CH2Cl2 (40 mL) to give a white suspension. To this at room temperature was added trimethylsilyl cyanide (40 mmol, 2.0 equiv.), followed by dropwise addition of alcohol (20 mmol, 1.0 equiv.) to give a moderate exothermic reaction. The mixture slowly turned clear, and TLC showed mostly the desire product. Saturated NaHCO3 was added and the mixture was concentrated in vacuo to remove the volatiles. The residue was partitioned between saturated NaHCO3 and ethyl acetate, and the aqueous layer was extracted one more time with ethyl acetate. Combined organic layers were dried over MgSO4, filtered and concentrated. The crude product was purified by flash column chromatography on silica gel (5% ethyl acetate/hexanes) to give the desired Diphenylacetonitrile.
Reactions
Methadone is synthesised by alkylation of diphenylacetonitrile using 2-dimethylaminopropylchloride in the presence of sodamide.
Dextromoramide is synthesised by alkylation of diphenylacetonitrile using 1-morpholinyl-2- chloropropane in the presence of sodamide.
Diphenylacetonitrile in the presence of sodium amide is alkylated with 1-ethyl-3-chlorpyrrolidine, giving (1-ethyl-3-pyrrolidinyl) diphenylacetonitrile.
Isopropamide, is synthesized by alkylating diphenylacetonitrile with di-isopropylaminoethylchloride in the presence of sodium amide, and the subsequent hydrolysis of the nitrile group of the resulting compound to an amide group.
Synthesis Reference(s)
The Journal of Organic Chemistry, 35, p. 3253, 1970 DOI: 10.1021/jo00835a016
Safety Profile
Poison by ingestion, intraperitoneal, and intravenous routes. Moderately toxic by subcutaneous route. Questionable carcinogen with experimental carcinogenic and tumorigenic data. When heated to decomposition it emits toxic fumes of NOx, and CN-. See also NITRILES.
Synthesis
Add the 250mL ethyl acetate in 500mL glass there-necked flask, open and stir, add the 150g phenylcarbinol, add sodium methylate 80g, 70 ℃ of stirring reactions 2 hours are down to room temperature, add benzyl cyanide 120ml.Be heated to 110 ℃, distillation reaction 10h.Reaction naturally cools to room temperature after finishing, and adds the extraction of 200ml ethyl acetate and 200ml water, tells lower aqueous layer.The upper strata ethyl acetate layer adds water, and washing once adds anhydrous sodium sulfate drying.Concentrating under reduced pressure is removed ethyl acetate.Then in oily matter, add a small amount of dehydrated alcohol crystallisation by cooling, the crystallized product oven dry.Yield 90%, purity 99.0% (GC).
Description
Diphenylacetonitrile can be prepared in good yield by the dehydration of the amide of diphenyl acetic acid with phosphorous oxychloride.
What are the applications of Application
Diphenylacetonitrile undergoes anhydrous condensation with ethyl-4-bromo-butyrate. This is followed by its hydrolysis in alkaline medium that is used in the quantitative determination of 3-cyano-3,3-diphenylpropionic acid.
Purification Methods
Crystallise the nitrile from EtOH or pet ether (b 90-100o). [Beilstein 9 H 674, 9 IV 2505.]
Properties of Diphenylacetonitrile
| Melting point: | 71-73 °C (lit.) |
| Boiling point: | 181 °C/12 mmHg (lit.) |
| Density | 1.1061 (rough estimate) |
| vapor pressure | 21.3 hPa (190 °C) |
| refractive index | 1.5850 (estimate) |
| Flash point: | 120 °C |
| storage temp. | Store below +30°C. |
| solubility | INSOLUBLE |
| form | Crystalline Powder |
| color | White to creamy or faint yellow |
| Water Solubility | INSOLUBLE |
| Merck | 14,3316 |
| BRN | 1911160 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| CAS DataBase Reference | 86-29-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzeneacetonitrile, «alpha»-phenyl-(86-29-3) |
| EPA Substance Registry System | Diphenylacetonitrile (86-29-3) |
Safety information for Diphenylacetonitrile
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Diphenylacetonitrile
| InChIKey | NEBPTMCRLHKPOB-UHFFFAOYSA-N |
Diphenylacetonitrile manufacturer
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

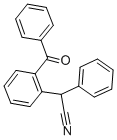
![2-[CYANO(PHENYL)METHYL]BENZENECARBONITRILE](https://img.chemicalbook.in/CAS/GIF/127667-03-2.gif)
![2-[(2-CHLORO-6-FLUOROPHENYL)(CYANO)METHYL]BENZENECARBONITRILE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6285605.gif)
You may like
-
 Diphenylacetonitrile 98%View Details
Diphenylacetonitrile 98%View Details -
 Diphenylacetonitrile 98%View Details
Diphenylacetonitrile 98%View Details -
 Diphenylacetonitrile 98%View Details
Diphenylacetonitrile 98%View Details -
 Diphenylacetonitrile CAS 86-29-3View Details
Diphenylacetonitrile CAS 86-29-3View Details
86-29-3 -
 Diphenylacetonitrile pure CAS 86-29-3View Details
Diphenylacetonitrile pure CAS 86-29-3View Details
86-29-3 -
 Diphenylacetonitrile pure LR, Cas Number: 86-29-3, DipanView Details
Diphenylacetonitrile pure LR, Cas Number: 86-29-3, DipanView Details
86-29-3 -
 Diphenyl Acetonitrile DPAN, Cas Number: 86-29-3View Details
Diphenyl Acetonitrile DPAN, Cas Number: 86-29-3View Details
86-29-3 -
 Diphenyl Acetonitrile, C14H11N, Cas Number: 86-29-3View Details
Diphenyl Acetonitrile, C14H11N, Cas Number: 86-29-3View Details
86-29-3
