DIMETHYLANILINE
- CAS NO.:1300-73-8
- Empirical Formula: C48H66N6
- Molecular Weight: 727.08
- MDL number: MFCD00167021
- EINECS: 215-091-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
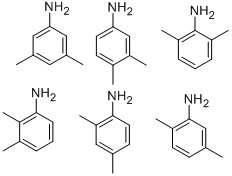
What is DIMETHYLANILINE?
Description
There are six xylidine isomers. Xylidine,mixed isomers (principally made up of 2,4-, 2,5-, and 2,6-isomers), is a pale yellow to brown liquid with a weak,aromatic amine odor. The odor threshold is 0.056 ppm.Molecular weight=121.20; Specific gravity (H2O:1) =0.98 at 25℃; Boiling point (mixed isomers) =213-226℃;Freezing/Melting point=236℃; Vapor pressure =<1 mmHg at 25℃; Flash point (2,6-isomer)= 91℃;(mixed isomers) 96.7℃. Explosive limits (2,6-isomer):LEL =1.0%; UEL—unknown. Hazard Identification (basedon NFPA-704 M Rating System): Health 3, Flammability 1,Reactivity 0. Insoluble in water.
Chemical properties
Liquid. Slightly soluble inwater; soluble in alcohol and ether. Combustible.
Chemical properties
There are six xylidine isomers. Xylidine, mixed isomers (principally made up of 2,4-, 2,5-, and 2,6-isomers) is a pale yellow to brown liquid with a weak, aromatic amine odor.
The Uses of DIMETHYLANILINE
Chiefly in the manufacture of dyes.
The Uses of DIMETHYLANILINE
Chemical intermediate in the manufacture of pesticides, dyes, antioxidants, pharmaceuticals, synthetic resins, and fragrances.
Definition
A varying mixture of isomers (2,3-; 2,4-; 2,5-; 2,6-).
Hazard
Toxic by ingestion, inhalation, and skin absorption. Liver damage. Methemoglobinemia. Possible carcinogen.
Health Hazard
Xylidine causes liver damage in experimental animals and is a mild methemoglobin former; it caused tumors of the nasal cavity in rats. There are six isomeric forms of xylidenes with the commercial product consisting primarily of the 2,4- and 2,6-isomers.
Safety Profile
Confirmed carcinogen. Poison by intravenous route. Moderately toxic by ingestion. This material, which so closely resembles aniline in the character of its toxic effects, is actually twice as toxic as aniline. It can cause injury to the blood and the liver. It does not necessarily give any alarm or warning, such as cyanosis, headache, and duziness, whch characterize aniline poisoning. Thus, it may be considered a more insidious poison than aniline, and severe and possibly fatal intoxication may come about through skin absorption. Combustible when exposed to heat or flame. Can react vigorously with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx. See also ANILINE and other xylidme entries.
Potential Exposure
Xylidines are used in dyestuff manufacture; as intermediates in the manufacture of pesticides, antioxidants, pharmaceuticals, and other organic com pounds.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia. Spectrophotometry may be required for precise determination oflevels of methemoglobin in urine. If symptoms of seriouscyanosis develop, methylene blue may be given as anantidote (by a trained medical person only), over 5 min.Repeat in 1 h if not improving. 100% oxygen can be givenonly by a trained person.
Carcinogenicity
The IARC has determined that there is sufficient evidence for the carcinogenicity of 2,6-xylidine in experimental animals and inadequate evidence in humans.5 Overall, 2,6- xylidine is considered possibly carcinogenic to humans. In genotoxic assays, 2,6-xylidine induced sister chromatid exchanges and chromosomal aberrations in cultured mammalian cells but did not induce micronuclei in the bone marrow of mice treated in vivo; conflicting results have been reported in the Salmonella typhimurium assay.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Before entering confined space where this chemicalmay be present, check to make sure that an explosiveconcentration does not exist. Xylidine must be stored toavoid contact with strong oxidizers (such as bromine,chlorine, or fluorine) since violent reactions occur. Contactwith hypochlorite bleaches may form explosive chloroamines. Store in tightly closed containers in a cool, dry,well-ventilated area away from heat sources. Sources ofignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard. Metalcontainers involving the transfer of 5 gallons or more ofthis chemical should be grounded and bonded. Drums mustbe equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof this chemical. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045.
Shipping
UN1711/Xylidines, solid or liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Vapor may form explosive mixture with air. Contact with hypo chlorite salts and bleaches form explosive chloroamines. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. A chemical base: Will neutralize acids to form salts plus water with an exothermic reaction. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents such as hydrides, nitrides, alkali metals, and sulfides.
Waste Disposal
Incineration; oxides of nitrogen are removed from the effluent gas by scrubber, catalytic, or thermal device.
Properties of DIMETHYLANILINE
| Melting point: | -36°C |
| Boiling point: | bp 213-226° |
| Density | 0,98 g/cm3 |
| refractive index | 1.4780 (estimate) |
| Flash point: | 97°C |
| form | Liquid, except o-4-xylidine
is a solid. |
| Merck | 14,10084 |
| Dielectric constant | 4.4(20℃) |
| CAS DataBase Reference | 1300-73-8(CAS DataBase Reference) |
| EPA Substance Registry System | Xylidine (1300-73-8) |
Safety information for DIMETHYLANILINE
Computed Descriptors for DIMETHYLANILINE
DIMETHYLANILINE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Xylidine, isomer mixture 98%View Details
Xylidine, isomer mixture 98%View Details -
 1300-73-8 99%View Details
1300-73-8 99%View Details
1300-73-8 -
 Xylidine, isomer mixture 98%View Details
Xylidine, isomer mixture 98%View Details
1300-73-8 -
 Xylidine (mixture) CAS 1300-73-8View Details
Xylidine (mixture) CAS 1300-73-8View Details
1300-73-8 -
 99.5% Xylidine Chemical PowderView Details
99.5% Xylidine Chemical PowderView Details
1300-73-8 -
 Dimethylaniline CAS 1300-73-8View Details
Dimethylaniline CAS 1300-73-8View Details
1300-73-8 -
 XYLIDINEView Details
XYLIDINEView Details
1300-73-8 -
 Analytical Grade Dimethylaniline Compound, 99%, 121-69-7View Details
Analytical Grade Dimethylaniline Compound, 99%, 121-69-7View Details
121-69-7
