2-(Dimethylamino)ethyl acrylate
- CAS NO.:2439-35-2
- Empirical Formula: C7H13NO2
- Molecular Weight: 143.18
- MDL number: MFCD00038233
- EINECS: 219-460-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
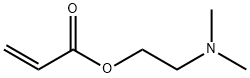
What is 2-(Dimethylamino)ethyl acrylate?
The Uses of 2-(Dimethylamino)ethyl acrylate
2-(Dimethylamino)ethyl acrylate (AmAc) are aminoacrylates. AmAc was used to fabricate gold/acrylic polymer nanocomposites.1 2-(dimethylamino)ethyl acrylate (DAEA) can undergo copolymerization with 2-acrylamido-2-methylpropanesulphonic acid (AMPS).
What are the applications of Application
2-(Dimethylamino)ethyl acrylate is an important raw material and intermediate used in organic synthesis, pharmaceuticals, as a catalytic agent, as a petrochemical additive and agrochemicals.
General Description
A colorless to light yellow liquid with an acrid odor. Insoluble in water and floats on water. Irritates the eyes and produces tears.
Air & Water Reactions
Highly flammable. Insoluble in water. Dimethylaminoethyl acrylate hydrolyzes slowly in water to produce acrylic acid and dimethylaminoethanol.
Reactivity Profile
Lachrymater. The reactive groups, in this substance, are the amine and ester and their possible reactivity concerns are listed below: Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Dimethylaminoethyl acrylate is flammable.
Flammability and Explosibility
Non flammable
Properties of 2-(Dimethylamino)ethyl acrylate
| Melting point: | -60°C |
| Boiling point: | 64 °C12 mm Hg(lit.) |
| Density | 0.943 g/mL at 25 °C(lit.) |
| vapor pressure | 0.35 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 138 °F |
| storage temp. | 2-8°C |
| form | clear liquid |
| pka | 8.21±0.28(Predicted) |
| color | Colorless to Light yellow to Light orange |
| Water Solubility | Soluble in water (1.000 g/L at 20°C). |
| Sensitive | Light Sensitive |
| BRN | 1099119 |
| CAS DataBase Reference | 2439-35-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propenoic acid, 2-(dimethylamino)ethyl ester(2439-35-2) |
| EPA Substance Registry System | 2-(Dimethylamino)ethyl acrylate (2439-35-2) |
Safety information for 2-(Dimethylamino)ethyl acrylate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-(Dimethylamino)ethyl acrylate
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2-(Dimethylamino)ethyl Acrylate (stabilized with MEHQ) CAS 2439-35-2View Details
2-(Dimethylamino)ethyl Acrylate (stabilized with MEHQ) CAS 2439-35-2View Details
2439-35-2 -
 2-(Dimethylamino)ethyl acrylate, stabilized with ≈0.1% 4-methoxyphenol CAS 2439-35-2View Details
2-(Dimethylamino)ethyl acrylate, stabilized with ≈0.1% 4-methoxyphenol CAS 2439-35-2View Details
2439-35-2 -
 2-(Dimethylamino)ethyl acrylate CAS 2439-35-2View Details
2-(Dimethylamino)ethyl acrylate CAS 2439-35-2View Details
2439-35-2 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0