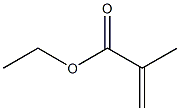
Ethyl methacrylate
Synonym(s):2-Methyl-2-propenoic acid;Ethyl 2-methylpropenoate, Methacrylic acid ethyl ester;Ethyl methacrylate
- CAS NO.:97-63-2
- Empirical Formula: C6H10O2
- Molecular Weight: 114.14
- MDL number: MFCD00009161
- EINECS: 202-597-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:41
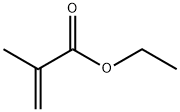
What is Ethyl methacrylate?
Description
Ethyl Methacrylate is the ester of ethyl alcohol and methacrylic acid. Other chemical names for this compound are Ethyl 2- Methyl-2-Propenoate; 2-Methyl-2-Propenoic Acid, Ethyl Ester; 2-Propenoic Acid, 2-Methyl-, Ethyl Ester; and Methyacrylic Acid, Ethyl Ester. It is a colorless liquid with a melting point below -75℃, a boiling point of 119℃, and a specific gravity of 0.911. It has a molecular weight of 114.14 Da, a refractive index (n, 25/D) of 1.4116, and a flash point (OC) of 70℃. Ethyl Methacrylate has an acrid acrylate odor and is soluble in alcohol and ether. It is readily polymerized and is chemically reactive. Ethyl Methacrylate monomer is very hydrophobic and poorly “wets” the hydrophilic keratin surface of the nail plate, requiring hydrophilic nail primers and additives to obtain sufficient adhesion[1].
References
[1] Andersen, F. A. “Amended Final Report on the Safety Assessment of Ethyl Methacrylate.” International Journal of Toxicology 21 1 (2002): 63–79.
Chemical properties
colourless liquid with an unpleasant odour
Chemical properties
Ethyl methacrylate is a clear, colorless, and highly flammable liquid that polymerizes but at a slower rate than that of the parent acrylate.
The Uses of Ethyl methacrylate
Ethyl methacrylate can be manufactured via a reaction of methacrylic acid or methyl acrylate with ethanol. It is used primarily for manufacturing polymers and as a component of acrylic polymers for surface coatings and as a structural monomer for some artificial fingernail formulations.
The Uses of Ethyl methacrylate
Polymers, chemical intermediates.
The Uses of Ethyl methacrylate
Ethyl methacrylate is used in dental protheses or in photobonded sculptured nails.
Production Methods
Ethyl methacrylate can be manufactured via a reaction of methacrylic acid or methyl acrylate with ethanol.
General Description
A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions
Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile
May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Hazard
Flammable, dangerous fire and explosion hazard. An irritant.
Health Hazard
Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard
Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Flammability and Explosibility
Non flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: If proper concentration of inhibitor is not present or when material is hot, a violent polymerization reaction may occur; Inhibitor of Polymerization: Oxygen in the air inhibits polymerization.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Mildly toxic by inhalation. Experimental teratogenic and reproductive effects. A skin irritant. A very dangerous fire and explosion hazard when exposed to heat, sparks, or flame; can react with oxidzing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Carcinogenicity
Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.
Purification Methods
Wash the ester successively with 5% aqueous NaNO2, 5% NaHSO3, 5% NaOH, then water. Dry it over MgSO4, add 0.2% (w/w) of phenyl-.-naphthylamine, and distil it through a short Vigreux column (p 11) [Schultz J Am Chem Soc 80 1854 1958]. [Beilstein 2 IV 1523.]
Properties of Ethyl methacrylate
| Melting point: | -75 °C |
| Boiling point: | 118-119 °C (lit.) |
| Density | 0.917 g/mL at 25 °C (lit.) |
| vapor density | >3.9 (vs air) |
| vapor pressure | 15 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 60 °F |
| storage temp. | 2-8°C |
| solubility | 5.1g/l |
| form | Liquid |
| color | Clear colorless |
| Odor | Acrid acrylic. |
| explosive limit | 1.8%(V) |
| Water Solubility | 4 g/L (20 ºC) |
| BRN | 471201 |
| Stability: | Polymerizes in the presence of light or heat. Incompatible with peroxides, oxidizing agents, bases, acids, reducing agents, halogens and amines. Flammable. |
| CAS DataBase Reference | 97-63-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propenoic acid, 2-methyl-, ethyl ester(97-63-2) |
| EPA Substance Registry System | Ethyl methacrylate (97-63-2) |
Safety information for Ethyl methacrylate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethyl methacrylate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Ethyl methacrylate, 99% CAS 97-63-2View Details
Ethyl methacrylate, 99% CAS 97-63-2View Details
97-63-2 -
 Ethyl Methacrylate (stabilized with HQ) CAS 97-63-2View Details
Ethyl Methacrylate (stabilized with HQ) CAS 97-63-2View Details
97-63-2 -
 Ethyl methacrylate CAS 97-63-2View Details
Ethyl methacrylate CAS 97-63-2View Details
97-63-2 -
 Ethyl methacrylate CAS 97-63-2View Details
Ethyl methacrylate CAS 97-63-2View Details
97-63-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
