Dimethocaine
- CAS NO.:94-15-5
- Empirical Formula: C16H26N2O2
- Molecular Weight: 278.39
- MDL number: MFCD01697030
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-24 21:15:16
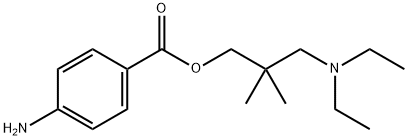
What is Dimethocaine?
Description
Dimethocaine, also known as DMC or larocaine, is a compound with stimulating effects similar to those of cocaine. It was used in the 1930s as a local anesthetic in dentistry and ophthalmology. Just like cocaine, dimethocaine is addictive due to its stimulation of the reward pathway in the brain. However, dimethocaine is a legal cocaine replacement in some countries and is even listed by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) under the category “synthetic cocaine derivatives”. The structure of dimethocaine, being a 4-aminobenzoic acid ester, resembles that of procaine.
Chemical properties
Dimethocaine is a yellow chrystalline powder. It is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas.
The Uses of Dimethocaine
Dimethocaine is a local anesthetic with stimulatory properties that is half the potency of cocaine. It is often abused as a legal substitute for cocaine. The drug is administered intravenously or nasally, because ingestion would lead to rapid hydrolyzation.Its positive effects are euphoria, stimulation, increased talkativeness and mood lift.However, because dimethocaine acts similar as cocaine, it has comparable negative side effects. These side effects include: tachycardia, difficulty with breathing, pain on the chest, vasoconstriction, insomnia, paranoia and anxiety.
Definition
ChEBI: Dimethocaine is a benzoate ester. It is derivate from cocaine, it is not a part of the organic alkaloid mix coming from a coca plant. It is very popular research chemical, mainly used as local anesthetic, but also the stimulant qualities appeared to be shown immediately.
Biological Activity
Dimethocaine is a local anesthetic that, because of its similarity in action to cocaine, has potential for abuse. This compound completely inhibits dopamine uptake in rat striatal synaptosomes with an IC50 value of 1.2 μM comparable to that of cocaine (IC50 = 0.7 μM). As a result, dimethocaine dose-dependently substitutes for cocaine in drug discrimination tests in rats and rhesus monkeys. This product is intended for forensic and research purposes.
Metabolism
Dimethocaine (DMC), a synthetic derivative of cocaine, is distributed and consumed as "new psychoactive substance" (NPS). It is mainly metabolized by N-acetylation, N-deethylation or hydroxylation. As DMC is metabolized via two main steps and different P450 isoforms were involved in the hepatic clearance of DMC, a clinically relevant interaction with single P450 inhibitors should not be expected. However, a slow acetylation phenotype or inhibition of NAT2 could lead to decreased N-acetylation and hence leading to an increased risk of side effects caused by this arylamine. Using the relative activity factor approach, the net clearances for deethylation of DMC were calculated to be 3% for P450 1A2, 1% for 2C19,<1% for 2D6, and 96% for 3A4. The net clearances for hydroxylation of DMC were calculated to be 32% for P450 1A2, 5% for 2C19, 51% for 2D6, and 12% for 3A4[1].
References
[1] Markus R Meyer, Hans H Maurer, Carina Lindauer. "Dimethocaine, a synthetic cocaine derivative: studies on its in vitro metabolism catalyzed by P450s and NAT2." Toxicology letters 225 1 (2014): 139–46.
Properties of Dimethocaine
| Melting point: | 53.0 to 57.0 °C |
| Boiling point: | 421.21°C (rough estimate) |
| Density | 1.035 |
| refractive index | 1.5290 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 10.08±0.25(Predicted) |
| color | Pale Orange to Light Orange |
| Merck | 14,3221 |
| InChI | InChI=1S/C16H26N2O2/c1-5-18(6-2)11-16(3,4)12-20-15(19)13-7-9-14(17)10-8-13/h7-10H,5-6,11-12,17H2,1-4H3 |
| CAS DataBase Reference | 94-15-5 |
Safety information for Dimethocaine
Computed Descriptors for Dimethocaine
| InChIKey | OWQIUQKMMPDHQQ-UHFFFAOYSA-N |
| SMILES | C(OC(=O)C1=CC=C(N)C=C1)C(CN(CC)CC)(C)C |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran

You may like
-
 Dimethocaine 98% CAS 94-15-5View Details
Dimethocaine 98% CAS 94-15-5View Details
94-15-5 -
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5