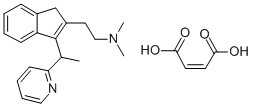
DIMETHINDENE
- CAS NO.:5636-83-9
- Empirical Formula: C20H24N2
- Molecular Weight: 292.42
- MDL number: MFCD00865676
- EINECS: 227-083-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
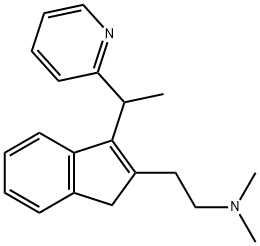
What is DIMETHINDENE?
Toxicity
As with other antihistaminic drugs, overdosage can produce the following symptoms: CNS depression accompanied by drowsiness (especially in adults), CNS stimulation and antimuscarinic effects (especially in children) including the following: excitation, ataxia, hallucinations, tonic or clonic spasms, mydriasis, dryness of the mouth, redness of the face, urine retention, fever and tachycardia. Blood hypotension is also possible. In its terminal phase, coma can be aggravated by cardiorespiratory colapse and death. There has been no report of a fatal outcome of Dimethindene overdosage.
Originator
Fenistil,Zyma,W. Germany,1961
Background
Dimetindene (Fenistil) is an antihistamine/anticholinergic used orally and locally as an antipruritic.
Indications
Indicated as symptomatic treatment of allergic reactions: urticaria, allergies of the upper respiratory tract such as hey fever and perennial rhinitis, food and drug allergies; pruritus of various origins, except pruritus due to cholestasis; insect bites. Dimethindene is also indicated for pruritus in eruptive skin diseases such as chicken-pox. Dimethindene can also be used as an adjuvant in eczema and other pruriginous dermatoses of allergic origin.
Definition
ChEBI: Dimetindene is an indene.
Manufacturing Process
26 grams of 2-ethylpyridine is added dropwise with cooling to 20°C and in an
atmosphere of nitrogen to a stirred solution of 650 ml of an 0.37 molar
solution of phenyl lithium in benzene. After two hours a solution of 10 grams
of 2-(2-dimethylaminoethyl)-indan-1-one in 50 ml of dry ether is added over
a period of five minutes while stirring and cooling to room temperature. After
standing for 24 hours the organo-lithium compounds are decomposed by the
addition of 50 ml of water with external cooling. After separating the water
phase from the organic solution, the latter is washed several times with 50 ml
of water, and then extracted with a mixture of 40 ml of concentrated
hydrochloric acid and 100 ml of water.
The acidic solution, containing the 2-(2-dimethylaminoethyl)-1-[1-(2-pyridyl)-
ethyl]-indan-1-ol is heated on the steam bath for thirty minutes to effect
dehydration to the desired indene derivative. The solution is cooled, made
strongly basic with an aqueous solution of ammonia and then extracted with
ether. The ether phase is dried over sodium sulfate, filtered, evaporated and
the residue distilled.
At 15 mm pressure the excess of 2-ethylpyridine is removed, at 120°C/0.5
mm some unreacted 2-(2-dimethylaminoethyl)-indene distills and at 165°-
175°C/0.5 mm the 2-(2-dimethylaminoethyl)-3-[1-(2-pyridyl)-ethyl]-indene is
collected. It may be converted to an aqueous solution of the dihydrochloride
by dissolving it in the appropriate amount of dilute hydrochloric acid.
To a solution of 1.0 gram of 2-(2-dimethylaminoethyl)-3[1-(2-pyridyl)-ethyl]-
indene in 10 ml of ethanol is added while stirring and heating 0.4 gram of
maleic acid. On cooling the 2-(2-dimethylaminoethyl)-3-[1-(2-pyridyl)-ethyl]-
indene maleate crystallizes, is filtered off, washed with a small amount of
ethanol and recrystallized from ethanol, MP 158°C.
Therapeutic Function
Antihistaminic
Pharmacokinetics
Dimethindene occurs as a racemic mixture. The (S)-(+)-dimethindene is a potent M2-selective muscarinic receptor antagonist (with lower affinity for M1, M3, and M4 muscarinic receptors). The (R)-(-)-enantiomer is the eutomer (responsible for bioactivity) for histamine H1 receptor binding.
Synthesis
Benzylmalonic acid is esterified with dihydropyran and reacted with sodium hydride and 2-dimethylaminoethyl chloride in toluene to give ditetrahydropyranyl-2-benzyl-2- (2-dimethylaminoethyl)malonate, which is cyclized with the help of polyphosphoric acid to 2-(2-dimethylaminoethyl)-indan-1-one. This compound reacts with 2-ethylpyridine and phenyllithium to give the carbinol, which is dehydrated by heating to dimetindene .
Metabolism
Not Available
Properties of DIMETHINDENE
| Melting point: | 50 - 53°C |
| Boiling point: | 424.35°C (rough estimate) |
| Density | 1.0073 (rough estimate) |
| refractive index | 1.5640 (estimate) |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.58±0.28(Predicted) |
| color | Pale Brown to Light Brown |
| Water Solubility | 238.6mg/L(37 ºC) |
Safety information for DIMETHINDENE
Computed Descriptors for DIMETHINDENE
DIMETHINDENE manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran

You may like
-
 5636-83-9 Dimetindene 98%View Details
5636-83-9 Dimetindene 98%View Details
5636-83-9 -
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5