DIMETHINDENE MALEATE
Synonym(s):N,N-Dimethyl-2-{3-[(RS)-1-(pyridine-2-yl)ethyl]-1H-inden-2-yl}ethanamin-(Z)-butendioate
- CAS NO.:3614-69-5
- Empirical Formula: C24H28N2O4
- Molecular Weight: 408.49
- MDL number: MFCD00072146
- EINECS: 222-789-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
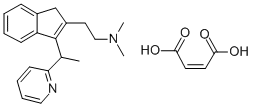
What is DIMETHINDENE MALEATE?
Chemical properties
White or almost white, crystalline powder.
Originator
Fenistil,Zyma,W. Germany,1961
The Uses of DIMETHINDENE MALEATE
Dimethindene Maleate is a salt of Dimethindene which is an antihistamine/anticholinergic used orally and locally as an antipruritic. Dimethindene is an H1-antagonist of high potency.
The Uses of DIMETHINDENE MALEATE
Antihistaminic.
Definition
ChEBI: Dimetindene maleate is an indene.
Manufacturing Process
26 grams of 2-ethylpyridine is added dropwise with cooling to 20°C and in an
atmosphere of nitrogen to a stirred solution of 650 ml of an 0.37 molar
solution of phenyl lithium in benzene. After two hours a solution of 10 grams
of 2-(2-dimethylaminoethyl)-indan-1-one in 50 ml of dry ether is added over
a period of five minutes while stirring and cooling to room temperature. After
standing for 24 hours the organo-lithium compounds are decomposed by the
addition of 50 ml of water with external cooling. After separating the water
phase from the organic solution, the latter is washed several times with 50 ml
of water, and then extracted with a mixture of 40 ml of concentrated
hydrochloric acid and 100 ml of water.
The acidic solution, containing the 2-(2-dimethylaminoethyl)-1-[1-(2-pyridyl)-
ethyl]-indan-1-ol is heated on the steam bath for thirty minutes to effect
dehydration to the desired indene derivative. The solution is cooled, made
strongly basic with an aqueous solution of ammonia and then extracted with
ether. The ether phase is dried over sodium sulfate, filtered, evaporated and
the residue distilled.
At 15 mm pressure the excess of 2-ethylpyridine is removed, at 120°C/0.5
mm some unreacted 2-(2-dimethylaminoethyl)-indene distills and at 165°-
175°C/0.5 mm the 2-(2-dimethylaminoethyl)-3-[1-(2-pyridyl)-ethyl]-indene is
collected. It may be converted to an aqueous solution of the dihydrochloride
by dissolving it in the appropriate amount of dilute hydrochloric acid.
To a solution of 1.0 gram of 2-(2-dimethylaminoethyl)-3[1-(2-pyridyl)-ethyl]-
indene in 10 ml of ethanol is added while stirring and heating 0.4 gram of
maleic acid. On cooling the 2-(2-dimethylaminoethyl)-3-[1-(2-pyridyl)-ethyl]-
indene maleate crystallizes, is filtered off, washed with a small amount of
ethanol and recrystallized from ethanol, MP 158°C.
brand name
Forhistal Maleate (Ciba-Geigy).
Therapeutic Function
Antihistaminic
General Description
Dimethindene maleate, (±)2-[1-[2-[2-dimethylamino)ethyl]-inden-3-yl]ethyl]pyridinebimaleate (1:1) (Forhistal Maleate), is a white to off-whitecrystalline powder that has a characteristic odor and is sparinglysoluble in water. This potent antihistaminic agent may beconsidered a derivative of the unsaturated propylamines. Theprincipal side effect is some sedation or drowsiness. The antihistaminicactivity resides mainly in the levorotatory isomer.
Properties of DIMETHINDENE MALEATE
| Melting point: | 159-161℃ |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Slightly soluble in water, soluble in methanol |
| form | neat |
| form | Solid |
| color | White to Pale Beige |
Safety information for DIMETHINDENE MALEATE
Computed Descriptors for DIMETHINDENE MALEATE
| InChIKey | SWECWXGUJQLXJF-BTJKTKAUSA-N |
| SMILES | C1(C(C)C2=NC=CC=C2)=C(CC2=CC=CC=C12)CCN(C)C.C(/C(=O)O)=C/C(=O)O |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
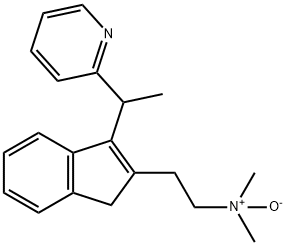
![DiMethindene IMpurity H (2-[(1RS)-1-(2-ethenyl-1H-Inden-3-yl)ethyl]pyridine)](https://img.chemicalbook.in/CAS/20180808/GIF/1346597-95-2.gif)
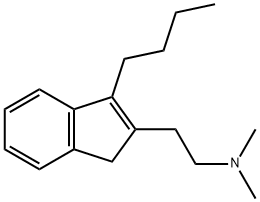
![α-[2-(DiMethylaMino)ethyl] HydrocinnaMic Acid Ethyl Ester](https://img.chemicalbook.in/CAS/GIF/92726-29-9.gif)
You may like
-
 Dimethindene maleate 98%View Details
Dimethindene maleate 98%View Details -
 3614-69-5 98%View Details
3614-69-5 98%View Details
3614-69-5 -
 Dimethindene maleate 98%View Details
Dimethindene maleate 98%View Details
3614-69-5 -
 Dimetindene maleate CAS 3614-69-5View Details
Dimetindene maleate CAS 3614-69-5View Details
3614-69-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
