DILAZEP DIHYDROCHLORIDE
- CAS NO.:20153-98-4
- Empirical Formula: C31H46Cl2N2O10
- Molecular Weight: 677.61
- MDL number: MFCD00133267
- EINECS: 243-548-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
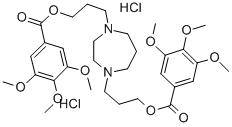
What is DILAZEP DIHYDROCHLORIDE?
Originator
Cormelian,Asta-Werke,W. Germany,1972
The Uses of DILAZEP DIHYDROCHLORIDE
Dilazep dihydrochloride has been used in estrogen receptor (ERα) antagonistic assay. It has also been used as an equilibrative nucleoside transporter inhibitor (ENT 1 and 2) in cancer cell lines.
The Uses of DILAZEP DIHYDROCHLORIDE
Dilazep Dihydrochloride is a research product for neuroscience. This compound may exhibit neuroprotective properties. Angiotensin converting, adenosine reuptake inhibitor. Antiplatelet agent. Dilazep acts as a cerebral vasodialator.
The Uses of DILAZEP DIHYDROCHLORIDE
Vasodilatator;Adenosine uptake inhibitor
What are the applications of Application
Dilazep dihydrochloride is an adenosine uptake inhibitor
Definition
ChEBI: Dilazep dihydrochloride is the dihydrochloride salt of dilazep. It inhibits adenosine uptake, ischemic damage, platelet aggregation, and membrane transport of nucleosides. It has a role as a cardioprotective agent, a platelet aggregation inhibitor and a vasodilator agent. It contains a dilazep(2+).
Manufacturing Process
528.8 grams of bis-(3-hydroxypropyl)-ethylene diamine [K. Schlgl and R.
Schlgl, Monatschefte der Chemie 95 (1964) page 935] are dissolved in a
mixture of 1,500 cc of anhydrous ethyl alcohol and 1,250 grams of
triethylamine. 520 grams of 1,3-chlorobromopropane are added thereto
dropwise over a period of about 3 hours while stirring and heating the
reaction mixture in an oil bath of 50°C. After completion of the addition, the
oil bath is heated to 60°C for 20 minutes while stirring of the reaction mixture
is continued. With increasing reaction time, triethylamine hydrochloride is
precipitated. After completion of the reaction, the mixture is allowed to cool to
room temperature.
Triethylamine hydrochloride is separated by filtration and the filter cake is
washed with 100 cc of anhydrous ethyl alcohol. The alcohol and the excess of
triethylamine is distilled off in a vacuum of a water pump. The residue
represents a light-yellowish brown viscous oil which is extracted 3 times with
500 cc of anhydrous benzene each time with stirring at 40° to 60°C. The
benzene is distilled off on a water bath at 60°C. Thus, an oil is obtained which
solidifies to a hard mass after some hours. This mass is crushed and dried over P2O5 in an exsiccator. The compound represents N,N'-bis-(3-
hydroxypropyl)homopiperazine. Yield: 128.5 grams. FP: 46°-47°C; BP0.02mm:
141°-142°C.
21.6 grams of N,N'-bis-(3-hydroxypropyl)homopiperazine obtained as
described and 63.8 grams of 3,4,5-trimethoxy benzoic acid chloride are
dissolved in 600 parts by volume of anhydrous chloroform. The solution is
heated to boiling for 5 hours. Thereafter, chloroform is distilled off in a
vacuum. The residue is dissolved in water and the aqueous solution is washed
with ether. Thereafter, the aqueous phase is rendered alkaline by the addition
of soda lye and the separated oil base is extracted with ether. The ethereal
solution is dried over Na2SO4. Ether is separated in a vacuum and the highly
viscous residue is dissolved in 150 parts by volume of ethyl alcohol. The
calculated equivalent amount of ethereal HCl is added thereto.
The soon crystallizing dihydrochloride is separated by filtration, dried and
recrystallized from 120 parts by volume of ethanol. Thus, after drying for 3
days over P2O5, 40-50 grams (66-70% of the theoretical) of N,N'-bis-[(3,4,5-
trimethoxy benzoloxy)propyl] homopiperazine dihydrochloride containing 1
mol of water of crystallization is obtained. This product has a melting point at
194°-198°C.
Therapeutic Function
Coronary vasodilator
General Description
Dilazep comprises central cyclic?diamine and phenyl rings connected via alkyl linkers.
Biochem/physiol Actions
Dilazep is a equilibrative nucleoside transporter 1 (ENTs) inhibitor. It is an antianginal drug and an effective coronary vasodilator. Dilazep elicits anti-albuminuric effects and is an anti-platelet agent. It is a potent adenosine uptake inhibitor and suppresses effects of ischemia.
storage
Store at RT
Properties of DILAZEP DIHYDROCHLORIDE
| Melting point: | 194-198° (monohydrate) |
| storage temp. | 2-8°C |
| solubility | H2O: 10 mg/mL |
| form | powder |
| color | white |
| Water Solubility | Soluble to 100 mM in water |
Safety information for DILAZEP DIHYDROCHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DILAZEP DIHYDROCHLORIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Dilazep dihydrochloride CAS 20153-98-4View Details
Dilazep dihydrochloride CAS 20153-98-4View Details
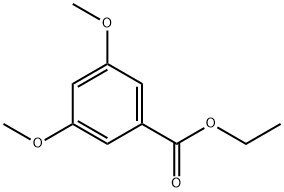
20153-98-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
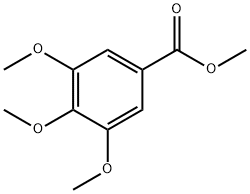
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
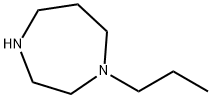
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
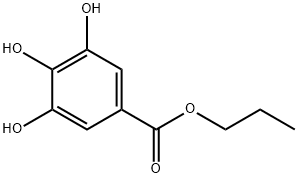
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1