Diisopropylammonium dichloroacetate
- CAS NO.:660-27-5
- Empirical Formula: C8H17Cl2NO2
- Molecular Weight: 230.13208
- MDL number: MFCD00868288
- EINECS: 211-538-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-05 20:24:49
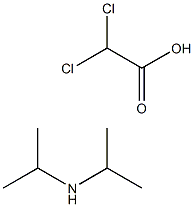
What is Diisopropylammonium dichloroacetate?
Description
Diisopropylammonium dichloroacetate is a hepatoprotective drug that improves the energy metabolism of hepatocytes, promotes the regeneration of injured hepatocytes, increases the rate of tissue cell respiration and oxygen respiration, and reduces the accumulation of fat in the liver. It is mainly used clinically for the treatment of fatty liver, intrahepatic cholestasis, and general liver dysfunction. It is also used in the treatment of acute and chronic hepatitis, hepatomegaly, and early cirrhosis.
Chemical properties
White or off-white loose mass or powder, slightly bitter, easily soluble in water, ethanol or chloroform, slightly soluble in ether.
History
In the 1950s, the compound diisopropylammonium dichloroacetate (DIPA) was used in the synthesis of methylated derivatives of a purportedly naturally occurring B vitamin (pangamic acid; dgluconodimethylaminoacetate). Anecdotal clinical reports appeared claimingefficacy in various metabolic and cardiovascular disorders from pharmaceuticalmixtures of pangamic acid and DIPA. In 1970,DCA was identified as themetabolically active moiety of DIPA(Stacpoole & Felts,1970) and it has beenused thereafter almost exclusively as the sodium salt.
The Uses of Diisopropylammonium dichloroacetate
Diisopropylammonium dichloroacetate (DIPA) is known to produce a significant and prolonged hypoglycemic effect in alloxan-diabetic but not in normal rats.
Diisopropylamine 2,2-Dichloroacetate is used in the treatment of antituberculosis drugs-induced liver injury.
Definition
ChEBI: Diisopropylamine dichloroacetate is an organohalogen compound and a carboxylic acid.
Synthesis
Diisopropylammonium dichloroacetate is prepared by the reaction of dichloro-acetic acid and diisopropylamine. The steps are as follows:
1 volume of acetone is mixed with 1.5 volumes of cyclohexane to obtain a mixed solvent; Add 1 mol of diisopropylamine (101.19g, C6H15N, Mr=101.19) into 150ml of mixed solvent, stir evenly, and heat the liquid material to 50°C; slowly add 1mol of dichloroacetic acid (128.94g, C2H2Cl2O2, Mr=128.94) dropwise to the liquid material obtained in step (at a speed of about 10g/min) under heat preservation and stirring conditions,After the feeding is complete, keep warm and continue to stir for 3 hours, then naturally cool to 4-8°C, and then stand at this temperature for 10 hours; Filter the material obtained in step, separate the mother liquor, collect white crystals, and dry at 70° C. to obtain 216.8 g of the diisopropylamine dichloroacetate (C8H17Cl2NO2, Mr=230.13), with a molar yield of 94.2%.
Mode of action
The mechanism of action of diisopropyl dichloroacetate is to enhance the fluidity of hepatocyte membranes by promoting sequential methylation of membrane phospholipids, to increase the activity of Na+-K+-ATPase, which is the main driving force of bile secretion and flow; to promote the functional repair of damaged hepatocytes, to improve the respiratory function and oxygen utilization of tissue cells, to increase the metabolic activity of fatty acids, to accelerate the oxidation of fatty acids, and to create conditions for the recovery of liver function.
Properties of Diisopropylammonium dichloroacetate
| Melting point: | 119-121° |
| RTECS | AG6475000 |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Crystalline Powder |
| color | White |
| Merck | 14,3197 |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C6H15N.C2H2Cl2O2/c1-5(2)7-6(3)4;3-1(4)2(5)6/h5-7H,1-4H3;1H,(H,5,6) |
| CAS DataBase Reference | 660-27-5 |
| EPA Substance Registry System | Acetic acid, dichloro-, compd. with N-(1-methylethyl)-2-propanamine (1:1) (660-27-5) |
Safety information for Diisopropylammonium dichloroacetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Diisopropylammonium dichloroacetate
| InChIKey | ILKBHIBYKSHTKQ-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C(Cl)Cl.CC(NC(C)C)C |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Diisopropylamine Dichloroacetate CAS 660-27-5View Details
Diisopropylamine Dichloroacetate CAS 660-27-5View Details
660-27-5 -
 Diisopropylammonium dichloroacetate 95.00% CAS 660-27-5View Details
Diisopropylammonium dichloroacetate 95.00% CAS 660-27-5View Details
660-27-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
