DIHYDROERGOTAMINE
- CAS NO.:511-12-6
- Empirical Formula: C33H37N5O5
- Molecular Weight: 583.69
- MDL number: MFCD00215847
- EINECS: 208-123-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:12:41
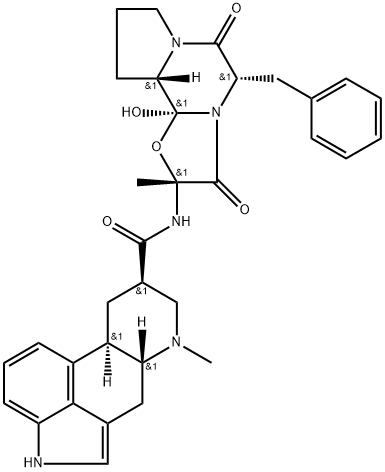
What is DIHYDROERGOTAMINE?
Absorption
When delivered intranasally, DHE has a bioavailability of approximately 40% with a tmax of 30-60 min.. Oral formulations have largely been unsuccessful due to a 1% bioavailability owing to a high degree of first-pass metabolism. Both intramuscular and intravenous administration produces 100% bioavailability with a tmax of 1-2 min and 24-34 min respectively.
Toxicity
Symptoms of overdose include numbness, tingling, pain, and cyanosis of the extremities associated with diminished or absent peripheral pulses; respiratory depression; an increase and/or decrease in blood pressure, usually in that order; confusion, delirium, convulsions, and coma; and/or some degree of nausea, vomiting, and abdominal pain. In case of overdose, warmth should be applied to affected areas and vasodilators administered. Local poison control centres should be contacted for detailed information on care in cases of ergot overdose.
The Uses of DIHYDROERGOTAMINE
Levadex is an intermediate in the synthesis of 2’-Epidihydroergotamine (E584910), which is an ergot alkaloid that exhibits potential neuroprotective properties against degenerative diseases.
The Uses of DIHYDROERGOTAMINE
Anti-adrenergic.
Indications
Dihydroergotamine (DHE) in all formulations is indicated for the acute treatment of migraine with or without aura in adults. As an injection, DHE is also indicated for the acute treatment of cluster headache episodes.
DHE is not indicated for migraine prevention or the management of hemiplegic or basilar migraine.
Background
A 9,10alpha-dihydro derivative of ergotamine. Dihydroergotamine is used as an abortive therapy for migraines. Its use has largely been supplanted by triptans in current therapy due to the class's greater selectivity and more favourable side effect profile.
Recent improvements have been made in the design of intranasal delivery devices allowing for greater delivery of dihydroergotamine solution to the vasculature-rich upper nasal cavity. The recently approved Precision Olfactory Delivery technology developed by Impel Neuropharma technology has correlated with an increase of 3-fold in Cmax and 4-fold in AUC despite the solution formulated at 75% of the strength of the existing intranasal product.
Definition
ChEBI: Ergotamine in which a single bond replaces the double bond between positions 9 and 10. A semisynthetic ergot alkaloid with weaker oxytocic and vasoconstrictor properties than ergotamine, it is used (as the methanesulfonic or tartaric acid salts) for the tr atment of migraine and orthostatic hypotension.
brand name
D.H.E. 45 (Valeant); Migranal (Valeant) .
Pharmacokinetics
DHE is indicated for the acute treatment of migraine headaches with or without aura and the acute treatment of cluster headache episodes. It is thought to exert its therapeutic effect through both neurological and vascular mechanisms. Its serotonin agonist activity may contribute to decreasing glutamatergic activity of the trigeminal system and subsequent cortical depolarization which is thought to participate in the neurological pathophysiology of migraine. The same serotonin agonist activity also contributes to vasoconstriction, producing both the characteristic side effect of chest tightness and potentially contributing to a therapeutic effect by counteracting the vasodilation due to calcitonin gene-related peptide (CGRP) release in migraine attacks.
Metabolism
DHE is metabolized in the liver to four identified metabolites. 8'-β-hydroxy dihydroergotamine is the primary metabolite and an active one with equipotency for adrenergic and 5-HT receptors. 8'-β-hydroxy dihydroergotamine is present at plasma concentrations 5-7 times that of DHE. The remaining metabolites are Dihydrolysergic acid, dihydrolysergic amide, and a fourth metabolite formed through oxidative opening of the proline ring are considered minor metabolites. After intranasal administration, it has been found that metabolites represent 20-30% of plasma AUC.
Properties of DIHYDROERGOTAMINE
| Melting point: | 239° |
| Boiling point: | 641.14°C (rough estimate) |
| alpha | D20 -64°; 20546 -79° (c = 0.5 in pyridine) |
| Density | 1.1992 (rough estimate) |
| refractive index | 1.7500 (estimate) |
| pka | 9≅+-.0.60(Predicted) |
Safety information for DIHYDROERGOTAMINE
Computed Descriptors for DIHYDROERGOTAMINE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1