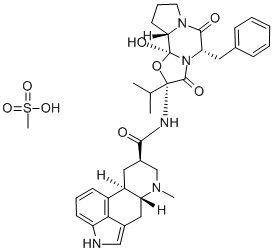
dihydroergocristine
- CAS NO.:17479-19-5
- Empirical Formula: C35H41N5O5
- Molecular Weight: 611.73
- MDL number: MFCD02751966
- EINECS: 241-493-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:12:30
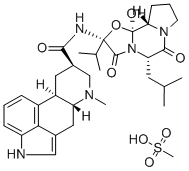
What is dihydroergocristine?
Absorption
Dihydroergocristine presents an absorption in the digestive tract of about 25% of the administered dose. When dihydroergocristine was orally administered in humans and the peak plasma concentration of 0.28 mcg/l was achieved after 0.46 hours. In the same report, the AUC was reported to be 0.39 mcg/l.h. To know more about dihydroergocristine as part of the ergoloid mesylate mixture, please visit Ergoloid mesylate.
Toxicity
Studies related to acute and chronic toxicity as well as teratogenesis and fertility has proven that dihydroergocristine is a non-toxic and very well tolerated drug. To know more about dihydroergocristine as part of the ergoloid mesylate mixture, please visit Ergoloid mesylate.
Background
Dihydroergocristine is part of the ergoloid mixture products. It is a semisynthetic ergot alkaloid and thus, it is characterized by a structural skeleton formed by an alkaloid ergoline. To know more about ergoloid mixtures, please visit Ergoloid mesylate.
Indications
Dihydroergocristine is used in some countries such as Brasil as a single agent for the treatment of cerebral and peripheric vascular events. To know more about dihydroergocristine as part of the ergoloid mesylate mixture, please visit Ergoloid mesylate.
Definition
ChEBI: Ergocristine in which a single bond replaces the double bond between positions 9 and 10. It is used as the mesylate salt for the symptomatic treatment of mental deterioration associated with cerebrovascular insufficiency and in peripheral vascular disease.
Pharmacokinetics
Dihydroergocristine has been shown to present effect on memory and cognition. This activity in the brain is been reported by an increase in glutathione in age-related brain states. The reported effect on serotonin and adrenergic receptors has also been correlated to an inhibition of platelet aggregation. It has also been reported that individuals exposed to dihydroergocristine may present an amphoteric vasoregulating activity either hypotensive in hypertensive individuals or hypertensive in hypotensive individuals. This action is performed by promoting a dilating action in the contracted arteries and a tonic action in the dilated arteries and arterioles. The vasoregulating effect causes an increase in cerebral blood flow and oxygen consumption by the brain, which correlates with the brain protective function of dihydroergocristine. In Alzheimer studies, dihydroergocristine reduced the amyloid-beta levels in different cell types. To know more about dihydroergocristine as part of the ergoloid mesylate mixture, please visit Ergoloid mesylate.
Metabolism
The major metabolite of dihydroergocristine is 8'-hydroxy-dihydroergocristine is produced in the liver. The modification of dihydroergocristine in the body is very extensive and it has been observed as an almost complete absence of the unchanged drug. To know more about dihydroergocristine as part of the ergoloid mesylate mixture, please visit Ergoloid mesylate.
Properties of dihydroergocristine
| Melting point: | 199℃ (decomposition) |
| Boiling point: | 899.3±65.0 °C(Predicted) |
| Density | 1.41±0.1 g/cm3(Predicted) |
| pka | pKa 6.89±0.07(H2O t=24 I≤0.01) (Uncertain) |
Safety information for dihydroergocristine
Computed Descriptors for dihydroergocristine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![Pyrrolo[1,2-a]pyrazine, octahydro-3-isobutyl- (5CI)](https://img.chemicalbook.in/CAS/GIF/718631-71-1.gif)
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4