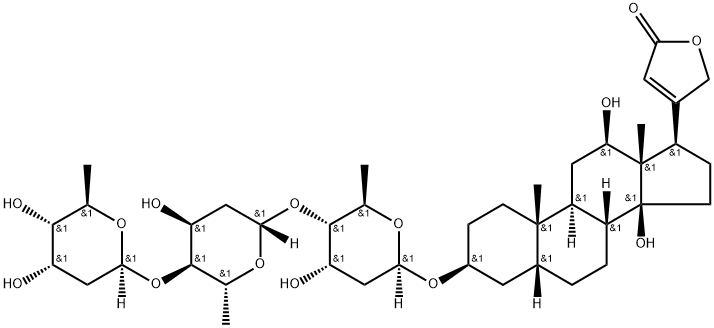
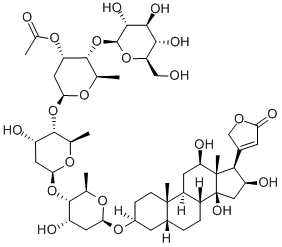
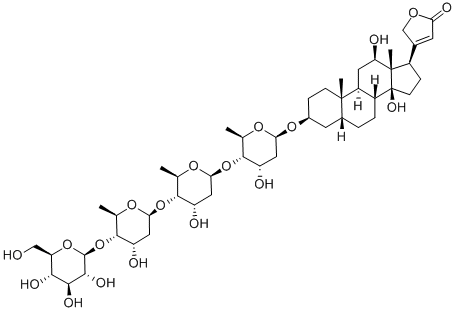
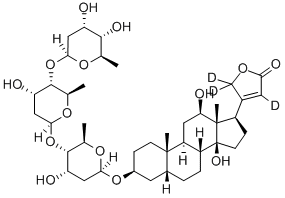
DIGOXIN
Synonym(s):12β-Hydroxydigitoxin;Digoxin
- CAS NO.:20830-75-5
- Empirical Formula: C41H64O14
- Molecular Weight: 780.95
- MDL number: MFCD00003674
- EINECS: 244-068-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
What is DIGOXIN?
Absorption
Digoxin is approximately 70-80% absorbed in the first part of the small bowel. The bioavailability of an oral dose varies from 50-90%, however, oral gelatinized capsules of digoxin are reported to have a bioavailability of 100%. Tmax, or the time to reach the maximum concentration of digoxin was measured to be 1.0 h in one clinical study of healthy patients taking 0.25 mg of digoxin with a placebo. Cmax, or maximum concentration, was 1.32 ± 0.18 ng/ml?1 in the same study, and AUC (area under the curve) was 12.5 ± 2.38 ng/ml?1.
If digoxin is ingested after a meal, absorption is slowed but this does not change the total amount of absorbed drug. If digoxin is taken with meals that are in fiber, absorption may be decreased.
A note on gut bacteria
An oral dose of digoxin may be transformed into pharmacologically inactive products by bacteria in the colon. Studies have indicated that 10% of patients receiving digoxin tablets will experience the degradation of at least 40% of an ingested dose of digoxin by gut bacteria. Several antibiotics may increase the absorption of digoxin in these patients, due to the elimination of gut bacteria, which normally cause digoxin degradation.
A note on malabsorption
Patients with malabsorption due to a variety of causes may have a decreased ability to absorb digoxin. P-glycoprotein, located on cells in the intestine, may interfere with digoxin pharmacokinetics, as it is a substrate of this efflux transporter. P-glycoprotein can be induced by other drugs, therefore reducing the effects of digoxin by increasing its efflux in the intestine.
Toxicity
Oral TDLO (human female): 100 ug/kg, Oral TDLO (human male): 75 ug/kg, Oral LD50 (rat): 28270 ug/kg
Digoxin toxicity can occur in cases of supratherapeutic dose ingestion or as a result of chronic overexposure. Digoxin toxicity may be manifested by symptoms of nausea, vomiting, visual changes, in addition to arrhythmia. Older age, lower body weight, and decreased renal function or electrolyte abnormalities lead to an increased risk of digoxin toxicity.
Description
Digoxin, 3β,14β-dihydroxy-5β-card-20(22)-enolide-3-rigitoxide, is also a glycoside isolated from various types of foxgloves. It differs from digitoxin in that it has an additional hydroxyl group at C16 of the steroid skeleton. It is extracted form the leaves of Digitalis lanta, Digitalis orientalis, or Scrophulariaceae.
Description
Digoxin is a secondary glycoside produced by plants in the Digitalis (foxglove) genus that were used for medicinal purposes as long ago as the 18th century. In 1930, Sydney Smith at Burroughs Wellcome (London) isolated it from one species, D. lanata.?
The US Food and Drug Administration approved the use of digoxin as a heart-disease medication in the late 1990s. It has been prescribed ever since under trade names such as Lanoxin for atrial fibrillation and heart failure. Digoxin, however, has a narrow therapeutic index; and drug interactions and adverse side effects are common. It has shown efficacy against some cancers, but other studies indicated that patients on digoxin have an increased risk of developing cancer.
Overdose is often fatal, as shown in the hazard information table. In 2003, a hospital nurse pleaded guilty to killing as many as 40 patients with digoxin and other heart medications.
Digoxin should not be confused with digitoxin1, which has one fewer hydroxyl group and is also a heart medication. For a new wrinkle on digoxin, see Molecule of the Future below.
1. CAS Reg. No. 71-63-6.
Description
Digoxin is a cardiac glycoside and metabolite of digitoxin that binds to and inhibits the Na+/K+-ATPase in cardiac tissues in an ATP- and Mg2+-dependent manner. This inhibition results in loss of the transmembrane Na+ gradient, which decreases activity of the Na+/Ca2+ exchanger, increasing intracellular Ca2+ levels, inotropy, and cardiac force. It increases activity of mitochondrial ATPase and actomyosin ATPase in rat hearts, which is directly correlated with increased myofibrillar contractile strength. In vivo, digoxin also decreases right atrial pressure and increases cardiac output in a canine model of congestive heart failure produced by pulmonary artery constriction. Formulations containing digoxin have been used to treat atrial fibrillation.
Chemical properties
White Crystalline Powder
Physical properties
Appearance: white crystals or crystalline powder, odorless. Solubility: easily dissolved in pyridine, slightly soluble in dilute alcohol, slightly soluble in chloroform, insoluble in water and ethyl ether. Specific optical rotation: +9.5 to +12.0°. Melting point: 248–250?°C.
Originator
Digoxin,Alexandria Co.
History
Digitalis, a kind of scrophulariaceae biennial or perennial herb, originating in
Europe in central and southern mountains, which was isolated from plants of the
genus Digitalis, has a long history of treating heart disease. Digitalis was also
recorded in Chinese Materia Medica and now is largely cultivated in China.
In clinical practice, the commonly used digitalis so far is known as the cardiac
glycoside. (In the twentieth century, this kind of structure was called a glucoside. In
the late twentieth century, domestic individual chemical researchers proposed
renaming it glycoside, identical to lanatoside k Huang Zhong). In 1930, a researcher from
Burroughs Wellcome pharmaceutical company named Sydney Smith successfully
isolated several steroid glycosides, including digoxin. The later production of
digoxin by Burroughs Wellcome’s successor company GlaxoSmithKline is called
LANOXIN, which is also called DIGITEK.? Since that time, digoxin has been
widely used in clinical practice to treat patients with CHF and atrial arrhythmia.
Now digoxin is still the basis of heart failure treatment, and in most cases it is one
of the preferred first-line drugs.
In 1954, researchers from the Institute of Materia Medica of the Chinese
Academy of Medical Sciences improved the method of extracting digitoxin, resulting in a simpler and 3.5 times higher yield than that of extracting digitoxin in
German pharmaceutical. In addition, the purity of domestic digitalis was
improved. The injection preparation was then produced and applied in clinical
practice.
Recently greatly advances have been made in research on the effects of cardiac
glycoside on cancer. Using high-throughput screening methods that have been
widely applied to the identification of new cardiac glycosides, researchers found
that cardiac glycoside can inhibit transplantation tumor growth in mice. This
research also helped in the development of the domestic pharmaceutical industry.
The Uses of DIGOXIN
Digoxin exhibits strong systolic action and slows heart rate. It is removed from the organism faster than digitoxin. It is used from chronic cardiac insufficiency in decompensated valvular disease of the heart, myocardium overload in arterial hypertension, tachycardia, ventricular fibrillation, and other analogous situations.
The Uses of DIGOXIN
Cardiotonic
The Uses of DIGOXIN
cardiac stimulant
Indications
Digoxin is indicated in the following conditions: 1) For the treatment of mild to moderate heart failure in adult patients. 2) To increase myocardial contraction in children diagnosed with heart failure. 3) To maintain control ventricular rate in adult patients diagnosed with chronic atrial fibrillation.
In adults with heart failure, when it is clinically possible, digoxin should be administered in conjunction with a diuretic and an angiotensin-converting enzyme (ACE) inhibitor for optimum effects.
Background
Digoxin is one of the oldest cardiovascular medications used today. It is a common agent used to manage atrial fibrillation and the symptoms of heart failure. Digoxin is classified as a cardiac glycoside and was initially approved by the FDA in 1954.
This drug originates from the foxglove plant, also known as the Digitalis plant, studied by William Withering, an English physician and botanist in the 1780s. Prior to this, a Welsh family, historically referred to as the Physicians of Myddvai, formulated drugs from this plant. They were one of the first to prescribe cardiac glycosides, according to ancient literature dating as early as the 1250s.
What are the applications of Application
12β-Hydroxydigitoxin is a cardiac glycoside and PGP substrate
Definition
ChEBI: A cardenolide glycoside that is digitoxin beta-hydroxylated at C-12. A cardiac glycoside extracted from the foxglove plant, Digitalis lanata, it is used to control ventricular rate in atrial fibrillation and in the management of congestive heart failure with atrial fibrillation, but the margin between toxic and therapeutic doses is small.
Indications
Digoxin is used for congestive heart failure (CHF), paroxysmal supraventricular tachycarelia, atrial fibrillation, and atrial flutter.
Manufacturing Process
It has long been known that digitalis leaves owe their physiological activity to the presence in them of certain glucosidal constituents. A method of preparation of a well-defined crystalline glucoside is described. The new glucoside is separated in the following manner. The total glucosides of the leaf of Digitalis lanata prepared by the usual methods (e.g. that of Keller and Fromme, Lehrbuch der Pharm. Chem., Schmidt) are stirred with acetone or methyl ethyl ketone in the cold using approximately two parts of acetone or methyl ethyl ketone to one part of the glucosidal mixture. After standing the sparingly soluble glucosides (A) are separated. The filtrate is fractionally precipitated with water until no more solid separates on further addition of water. The solid (B) is separated and the filtrate is saturated with salt when a further precipitate (C) is formed. This salt precipitate is dried and extracted in the cold with methyl or ethyl alcohol and the solution diluted with water. On standing crystals of the new glucoside separate. The glucoside is freed from more soluble glucosides by boiling with small quantities of chloroform, acetone or methylethyl ketone in which it is sparingly soluble and then crystallized by concentrating a solution in hot 80% methyl or ethyl alcohol. The glucoside can also be crystallized by the addition of water or ether to a solution of the substance in pyridine. Further quantities of the glucoside may be obtained by extracting the aqueous filtrate from salt precipitate (C) with cold chloroform. The chloroform extract after evaporation is stirred with acetone or methyl ethyl ketone and the sparingly soluble portion is further purified by boiling with small quantities of chloroform and the sparingly soluble portion crystallized as above described. The glucoside is also present in precipitate, which separates from the acetone or methyl ethyl ketone and in the fraction (B) precipitated by water from the acetone or methyl ethyl ketone solution. It may be separated from these solutions by fractional crystallization from hot dilute alcohol, followed by boiling the less soluble fractions with acetone, methyl ethyl ketone, or chloroform and crystallization of the sparingly soluble portions above described. The new glucoside digoxin crystallizes in stout plates; MP: at about 265°C. (decomp.); αD25 =+17.9°.
brand name
Lanoxin (GlaxoSmithKline).
Therapeutic Function
Cardiotonic
General Description
Clear to white crystals or white crystalline powder. Odorless. Used as a cardiotonic drug.
General Description
Digitalis (Crystodigin) is isolated fromD. lanata and D. purpurea among other Digitalis spp., and isthe chief active glycoside in digitalis leaf, with 1 mg digitoxinequal to 1 g of digitalis leaf therapy. In patients who missdoses, digitalis is very useful for maintenance therapy becauseof the longer half-life it provides. The longer durationand increased half-life are a result of the lack of the C-12hydroxy that is present in digoxin. In digoxin, this hydroxyplays two roles: (a) it serves as a site for metabolism, whichreduces the compound’s half-life; and (b) it gives more hydrophiliccharacter, which results in greater water solubilityand ease in renal elimination.
General Description
Digoxin (Lanoxin) is a purified digitalis preparationfrom D. lanata and represents the most widely useddigitalis glycoside. This wide use is primarily a result of itsfast onset and short half-life. Position 3 of the steroid is substitutedwith three digitoxose residues that, when removed,provide a genin or aglycone steroid that is still capable of receptorbinding but with altered pharmacokinetics.
Health Hazard
Material is a digitalis glycoside. Ingestion can cause death. Material is considered super toxic; probable human oral lethal dose is less than 5 mg/kg, a taste (less than 7 drops) for a 70 kg (150 lb.) person. Persons at risk include those taking drugs for thyroid and renal diseases. Quinidine and diuretics taken concurrently with DIGOXIN can be hazardous. It should be used with extreme care during pregnancy and in nursing mothers.
Fire Hazard
Avoid light.
Pharmacokinetics
Digoxin is a positive inotropic and negative chronotropic drug, meaning that it increases the force of the heartbeat and decreases the heart rate. The decrease in heart rate is particularly useful in cases of atrial fibrillation, a condition characterized by a fast and irregular heartbeat. The relief of heart failure symptoms during digoxin therapy has been demonstrated in clinical studies by increased exercise capacity and reduced hospitalization due to heart failure and reduced heart failure-related emergency medical visits. Digoxin has a narrow therapeutic window.
A note on cardiovascular risk
Digoxin poses a risk of rapid ventricular response that can cause ventricular fibrillation in patients with an accessory atrioventricular (AV) pathway. Cardiac arrest as a result of ventricular fibrillation is fatal. An increased risk of fatal severe or complete heart block is present in individuals with pre-existing sinus node disease and AV block who take digoxin.
Clinical Use
Supraventricular arrhythmias
Supraventricular arrhythmias
Safety Profile
A deadly poison by most routes. Human systemic effects by ingestion: anorexia, cardlac arrhythmias, nausea and vomiting, visual field changes, pulse rate decrease, fall in blood pressure. An experimental teratogen. When heated to decomposition it emits acrid and irritating fumes. See also DIGITALIS.
Potential Exposure
NaturalProduct; Reproductive Effector. Digoxin is used as a cardiotonic drug.
Drug interactions
Potentially hazardous interactions with other drugs
Angiotensin-II antagonists: concentration increased
by telmisartan
Anti-arrhythmics: concentration increased by
amiodarone, dronedarone and propafenone (half
maintenance dose of digoxin).
Antidepressants: concentration reduced by St John’s
wort - avoid.
Antifungals: increased toxicity if hypokalaemia
occurs with amphotericin; concentration increased
by itraconazole
Antimalarials: concentration possibly increased
by quinine, hydroxychloroquine and chloroquine;
increased risk of bradycardia with mefloquine
Antivirals: concentration increased by daclatasvir
Calcium-channel blockers: concentration increased
by diltiazem, lercanidipine, nicardipine, verapamil
and possibly nifedipine; increased risk of AV block
and bradycardia with verapamil.
Ciclosporin: concentration increased by ciclosporin.
Colchicine: possibly increased risk of myopathy
Diuretics: increased toxicity if hypokalaemia occurs;
concentration increased by spironolactone and
possibly potassium canrenoate.
Ticagrelor: concentration of digoxin increased
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Molecular weight: Digitoxin, 764.96; Digoxin, 780.96. Cardiac glycosides have a characteristic chemical structure called an aglycone ring. This is coupled with one or more sugars. The aglycone portion of the glycoside includes a steroid nucleus and a lactone ring at the C17 position. Hydroxyl groups, oriented to the beta position, are present at the C3 and C14 positions. Increases in numbers of hydroxyl groups cause an increase in polarity and decrease in lipid solubility. Increases in numbers of sugars also increase polarity and reduce lipid solubility. Sugars are attached to the steroid nucleus through a hydroxyl group at the C3 position and influences solubility, absorption, toxicity, and other pharmacological parameters.
Metabolism
About 13% of a digoxin dose is found to be metabolized in healthy subjects. Several urinary metabolites of digoxin exist, including dihydrodigoxin and digoxigenin bisdigitoxoside. Their glucuronidated and sulfated conjugates are thought to be produced through the process of hydrolysis, oxidation, and additionally, conjugation. The cytochrome P-450 system does not play a major role in digoxin metabolism, nor does this drug induce or inhibit the enzymes in this system.
Metabolism
Digoxin is mainly excreted unchanged in the urine by
glomerular filtration and tubular secretion; reabsorption
also occurs. Extensive metabolism has been reported in a
minority of patients Metabolites that have been detected
in the urine include digoxigenin, dihydrodigoxigenin,
the mono- and bisdigitoxosides of digoxigenin, and
dihydrodigoxin. Digoxigenin mono- and bisdigitoxosides
are known to be cardioactive whereas dihydrodigoxin is
probably much less active than digoxin.
In about 10% of patients there is considerable reduction
to cardio-inactive metabolites, chiefly dihydrodigoxin,
and 40% or more of a dose may be excreted in the urine
as dihydrodigoxin. Bacterial flora in the gastrointestinal
tract appear to be responsible for this metabolism and
antibacterials can reduce the process.a Excretion of digoxin is proportional to the glomerular
filtration rate. After intravenous injection 50-70% of the
dose is excreted unchanged.
Storage
Store at RT
Shipping
This compound falls in the DOT ID and ERGNumber: UN3249. Medicine, solid, toxic, n.o.s. categoryand requires a shipping label of “POISONOUS/TOXICMATERIALS.” It falls in Hazard Class 6.1 and PackingGroup III.
Purification Methods
Crystallise digoxin from aqueous EtOH, aqueous pyridine, EtOH/CHCl3, and dry it in a vacuum at 100o. The melting point depends on heating rate, but when placed in a bath at 260o and heated slowly it decomposes at 265o. In EtOH it has max at 220nm ( 12,800). [Smith J Chem Soc 508 1930, X-ray: Go et al. Cryst Struct Commun 8 149, 1031 1979, Beilstein 18/4 V 381.] HIGHLY TOXIC.
Toxicity evaluation
Digitalis glycosides inhibit the Na–K–ATPase pump, causing increased intracellular sodium, and loss of intracellular potassium and subsequent increase in intracellular calcium. This results in a positive inotropic effect and decreased rate of cardiac conduction through the sinoatrial and atrioventricular nodes.
Properties of DIGOXIN
| Melting point: | 248 °C |
| Boiling point: | 661.93°C (rough estimate) |
| alpha | 25Hg +13.4 to 13.8° (c = 10 in pyridine) |
| Density | 1.1110 (rough estimate) |
| refractive index | 12 ° (C=10, Pyridine) |
| Flash point: | 9℃ |
| storage temp. | Refrigerator |
| solubility | Soluble in dimethyl sulfoxide and methanol. |
| form | powder |
| appearance | white crystals or powder |
| pka | 13.50±0.70(Predicted) |
| color | White to Almost white |
| Water Solubility | 46.08mg/L(room temperature) |
| Merck | 14,3167 |
| BRN | 77011 |
| CAS DataBase Reference | 20830-75-5 |
| IARC | 2B (Vol. 108) 2016 |
| EPA Substance Registry System | Digoxin (20830-75-5) |
Safety information for DIGOXIN
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H331:Acute toxicity,inhalation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for DIGOXIN
DIGOXIN manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Digoxin extrapure CAS 20830-75-5View Details
Digoxin extrapure CAS 20830-75-5View Details
20830-75-5 -
 Digoxin CAS 20830-75-5View Details
Digoxin CAS 20830-75-5View Details
20830-75-5 -
 Digoxin CAS 20830-75-5View Details
Digoxin CAS 20830-75-5View Details
20830-75-5 -
 Digoxin 98% CAS 20830-75-5View Details
Digoxin 98% CAS 20830-75-5View Details
20830-75-5 -
 Digoxin 98% (HPLC) CAS 20830-75-5View Details
Digoxin 98% (HPLC) CAS 20830-75-5View Details
20830-75-5 -
 Digoxine BP/USP APIView Details
Digoxine BP/USP APIView Details
20830-75-5 -
 DIGOXIN CAS 20830-75-5View Details
DIGOXIN CAS 20830-75-5View Details
20830-75-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
