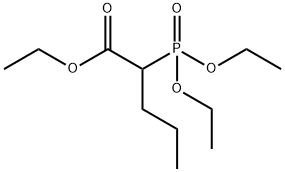
DIETHYLPHOSPHONOACETIC ACID
Synonym(s):(Diethoxyphosphinyl)acetic acid;Diethyl carboxymethylphosphonate;NSC 272281
- CAS NO.:3095-95-2
- Empirical Formula: C6H13O5P
- Molecular Weight: 196.14
- MDL number: MFCD00192032
- EINECS: 608-560-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-09-05 14:18:45
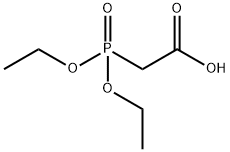
What is DIETHYLPHOSPHONOACETIC ACID?
Description
Diethylphosphonoacetic Acid (DPA) is a Horner-Wadsworth-Emmons (HWE) reagent. The mixed anhydride of trifluoroacetic acid—diethylphosphonoacetic acid Treat a 21-hydroxy-20-keto steroid leads directly to cardenolides by an intramolecular Horner-Emmons reaction. It could also used as a dummy template to prepare a molecularly imprinted polymer for use as an artificial receptor for organophosphorus pesticides (OPPs)[1-3].
Chemical properties
clear colorless to yellow viscous liquid
The Uses of DIETHYLPHOSPHONOACETIC ACID
Diethylphosphonoacetic Acid is a reagent that is used for enantioselective preparation of α-phosphoryl-α,β-unsatd.-δ-aryl-δ-lactones from nonracemic β-hydroxyaldehydes and their conversion to α-methylene-δ-aryl-δ-lactones.
The Uses of DIETHYLPHOSPHONOACETIC ACID
Acts as a nucleophile for nucleophilic addition reactions for synthesis of allene epoxides
Reactant for:
- Stereoselectivity studies of the Staudinger reaction
- Enantioselective formation of diols via epoxidation and hydration reactions
- Horner-Wadsworth-Emmons reactions
- Remote chelation controlled Ireland-Claisen rearrangement
- Ugi-Dieckmann reactions for synthesis of tetramic acid derivatives
What are the applications of Application
Diethylphosphonoacetic acid is a nucleophile for nucleophilic addition reactions
Synthesis Reference(s)
Journal of the American Chemical Society, 102, p. 4534, 1980 DOI: 10.1021/ja00533a047
References
[1] S. Donovan, J. Mcmurry, M. Avery. “Synthesis of digitoxigenin by remote functionalization.” Tetrahedron Letters 20 1 (1979): 3287–3290.
[2] A. M. Boldi, Hisham O. Eissa, Charles R. Johnson. “Solid-phase library synthesis of triazolopyridazines via [4+2] cycloadditions.” Tetrahedron Letters 40 1 (1999): 619–622.
[3] Lu Zhang. “Preparation and Characterization of Broad-Spectrum Artificial Antibody for OPPs Based on Dummy Template Imprinting Technique.” International Journal of Polymer Analysis and Characterization 117 1 (2014): 510–521.
Properties of DIETHYLPHOSPHONOACETIC ACID
| Boiling point: | 150 °C/0.05 mmHg (lit.) |
| Density | 1.220 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| form | Viscous Liquid |
| pka | 3.48±0.10(Predicted) |
| Specific Gravity | 1.220 |
| color | Clear colorless to yellow |
| InChI | InChI=1S/C6H13O5P/c1-3-10-12(9,11-4-2)5-6(7)8/h3-5H2,1-2H3,(H,7,8) |
Safety information for DIETHYLPHOSPHONOACETIC ACID
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DIETHYLPHOSPHONOACETIC ACID
| InChIKey | DVQMPWOLBFKUMM-UHFFFAOYSA-N |
| SMILES | C(O)(=O)CP(OCC)(OCC)=O |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine N-Boc-L-proline methyl ester 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazoleRelated products of tetrahydrofuran
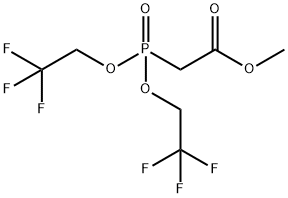
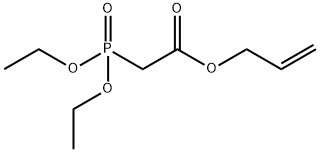
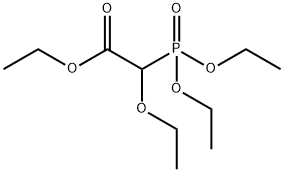
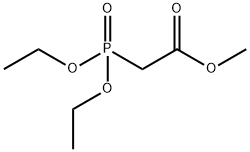
You may like
-
 Diethylphosphonoacetic acid 98% CAS 3095-95-2View Details
Diethylphosphonoacetic acid 98% CAS 3095-95-2View Details
3095-95-2 -
 Diethylphosphonoacetic acid CAS 3095-95-2View Details
Diethylphosphonoacetic acid CAS 3095-95-2View Details
3095-95-2 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5