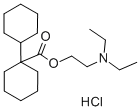
dicycloverine
- CAS NO.:77-19-0
- Empirical Formula: C19H35NO2
- Molecular Weight: 309.49
- MDL number: MFCD00599460
- EINECS: 201-009-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
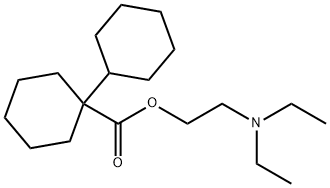
What is dicycloverine ?
Absorption
The bioavailability of dicyclomine has not been determined, though it is likely well absorbed as the primary route of elimination is in the urine. Dicyclomine has a Tmax of 1-1.5h.
Toxicity
Patients experiencing an overdose may present with headache, nausea, vomiting, blurred vision, dilated pupils, dizziness, dry mouth, difficulty swallowing, CNS stimulation, as well as hot, dry skin. Treat patients with gastric lavage, emetics, activated charcoal, sedatives for excitement, and a cholinergic agent if indicated.
The oral LD50 in mice is 625mg/kg.
Originator
Bentyl ,Merrell National ,US,1950
The Uses of dicycloverine
Anticholinergic.
Background
Dicyclomine is a muscarinic M1, M3, and M2 receptor antagonist as well as a non-competitive inhibitor of histamine and bradykinin used to treat spasms of the intestines seen in functional bowel disorder and irritable bowel syndrome. Though it is commonly prescribed, its recommendation may have been based on a small amount of evidence and so its prescription is becoming less favourable.
Dicyclomine was granted FDA approval on 11 May 1950.
Indications
Dicyclomine is indicated for the treatment of functional bowel disorder and irritable bowel syndrome.
Definition
ChEBI: The ester resulting from the formal condensation of 1-cyclohexylcyclohexanecarboxylic acid with 2-(diethylamino)ethanol. An anticholinergic, it is used as the hydrochloride to treat or prevent spasm in the muscles of the gastrointestinal tract, particularl that associated with irritable bowel syndrome.
Manufacturing Process
155 grams of 1-phenylcyclohexanecyanide, 350 cc of concentrated sulfuric
acid and 1,130 cc of ethyl alcohol are refluxed vigorously for 48 hours. The
remaining alcohol is then removed by vacuum distillation and the residue is
poured into 1 liter of ice water. An oil separates which is extracted 3 times
with 200 cc portions of petroleum ether, the extracts are combined and heated on a steam bath to remove the ether. The resulting crude ester may
be used directly for the reesterification operation or it may be distilled to
purify it first. A mixture of the ester so obtained, 155 grams of βdiethylaminoethanol and 800 cc of dry xylene are placed in a reaction vessel
with about 2 grams of sodium. The vessel is heated in an oil bath at 150°-
160°C. A xylene-ethanol azeotrope distills over at about 78°-82°C over a
period of 2 to 3 hours. The distillate is cooled and shaken with about 3 times
its volume of water, the decrease in volume of the distillate being considered a
measure of the amount of alcohol formed. When 80-90% of the theoretical
amount of alcohol is obtained in the distillate the reaction mixture is subjected
to vacuum distillation to remove most of the xylene and unreacted
diethylaminoethanol. The residue is poured into 500 cc of benzene which is
then extracted 3 times with 500 cc portions of water.
The washed benzene layer is diluted with an equal volume of ether and
alcoholic hydrochloric acid is added until the mixture is acid to Congo red. A
white crystalline solid forms which is dissolved in 300-400 cc of alcohol and
diluted with ether to the point where precipitation starts. A few drops of
butanone are added, the solution is cooled to -10°C, and filtered to recover
the crystals which separate. The product is obtained in the form of white
needles melting at 159°-160°C, in good yield.
13 parts of β-diethylaminoethyl 1-phenylcyclohexanecarboxylate
hydrochloride, 125 parts of glacial acetic acid and 0.3 part of Adams' catalyst
are heated to 70°C and shaken with hydrogen at 50 lb pressure until 90-
100% of the theoretical hydrogen is absorbed. The acetic acid is then
removed by distillation and the residue recrystallized from butanone, giving
the above product as a crystalline hydrochloride melting at 165°-166°C, in
good yields. This product may also be prepared by reacting cyclohexyl
bromide with cyclohexyl cyanide with the use of sodium amide followed by
alcoholysis and reesterification.
brand name
Bentyl (Axcan Scandipharm);Abacid plus;Ametil;Babypasmil;Babyspasmil;Baycyclomine;Benacol;Colix;Cyclobex;Cyclocen;Diarrest;Diclophen;Eatongel;Esentil;Fomulex;Formulex;Gastrosilane;Icramin;Incramin;Incron;Inctacol-c;Isospamex;Kolanticon;Lagasediv;Menospasm;Merbantal;Neoquess;Nomocramp;Notensyl;Or-tyl;Pamin;Panakiron;Prinel;Protylol;Sawamin;Spactil;Spasmoban;Spastil;Tarestin;Viscerol.
Therapeutic Function
Spasmolytic
World Health Organization (WHO)
Dicycloverine, an anticholinergic agent with antispasmodic and local anaesthetic activity, was introduced in 1952 for treatment of functional conditions involving smooth muscle of the gastrointestinal tract. Its use in the treatment of colic in infants under six months of age has been associated with irritability and restlessness, convulsions and apnoea which has led the major manufacturer to issue revised global prescribing information in 1985 contraindicating the use of dicycloverine in this age group. Subsequently restrictive regulatory action directed to other available brands of this drug was taken in several countries. Preparations containing dicycloverine remain available in at least ten major markets.
Pharmacokinetics
Dicyclomine is an anticholinergic drug used to relax the smooth muscles of the intestines. It's duration of action is not especially long as it is usually taken 4 times daily with individual doses of 20-40mg orally or 10-20mg by intramuscular injection. Dicyclomine should not be administered intravenously.
Metabolism
The metabolism of dicyclomine has not been well researched.
Properties of dicycloverine
| Boiling point: | 142-143 °C(Press: 0.5 Torr) |
| Density | 0.990±0.06 g/cm3(Predicted) |
| pka | 9.24±0.25(Predicted) |
| EPA Substance Registry System | [1,1'-Bicyclohexyl]-1-carboxylic acid, 2-(diethylamino)ethyl ester (77-19-0) |
Safety information for dicycloverine
Computed Descriptors for dicycloverine
dicycloverine manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
![[1,1'-bicyclohexyl]-1-carboxylic acid](https://img.chemicalbook.in/CAS/GIF/60263-54-9.gif)


You may like
-
 Dicyclomine 99%View Details
Dicyclomine 99%View Details
77-19-0 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2