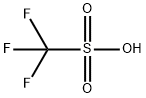
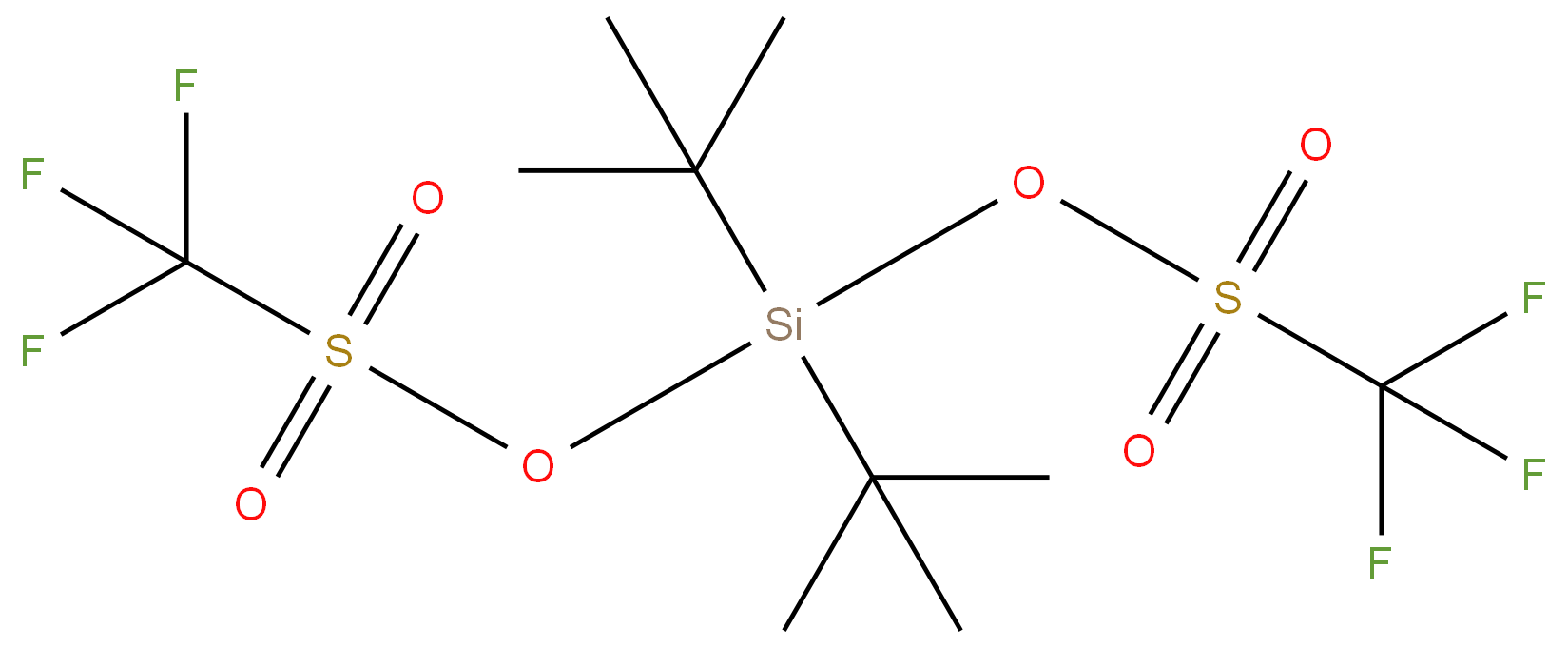
DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE)
Synonym(s):Di-tert-butylsilyl ditriflate;DTBS ditriflate;Trifluoromethanesulfonic acid di-tert-butylsilylene ester
- CAS NO.:85272-31-7
- Empirical Formula: C10H18F6O6S2Si
- Molecular Weight: 440.45
- MDL number: MFCD00010581
- EINECS: 680-172-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-12 17:17:06
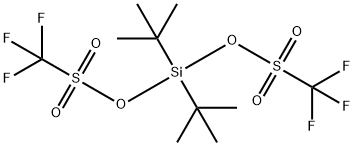
What is DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE)?
Chemical properties
Colorless to yellow liquid
Physical properties
bp 73–75°C/0.35 mmHg; d 1.208 g cm?3.
The Uses of DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE)
Di-t-butylsilyl bis(trifluoromethanesulfonate)
is a reagent for the selective protection of polyhydroxy
compounds. This reagent reacts with 1,2-, 1,3-, and 1,4-diols
under mild conditions to give the corresponding dialkylsilylene
derivatives in high yield (0–50°C, 79–96%). Deprotection is
conveniently achieved by using aqueous hydrofluoric acid in acetonitrile
(eq 1).
Unlike di-t-butyldichlorosilane, this reagent reacts with hindered
alcohols. Even pinacol reacts to give the silylene derivative
(100°C, 24 h, 70%). Di-t-butylsilylene derivatives of 1,2-diols are
more reactive than those of 1,3- and 1,4-diols and undergo rapid
hydrolysis (5 min) in THF/H2O at pH 10, while the 1,3- and 1,4-
derivatives are unaffected at pH 4–10 (22°C) for several hours.
This protecting group is stable under the conditions of PDC oxidation
of alcohols (CH2Cl2, 25?C, 27 h) and tosylation of alcohols
(pyridine, 25°C, 27 h).
The reagent has seen limited use for the protection of alcohols
but has been used to protect nucleosides (eq 2).The procedure
consists of sequential addition of the ditriflate and triethylamine
to the nucleoside in DMF. The choice of solvent is critical.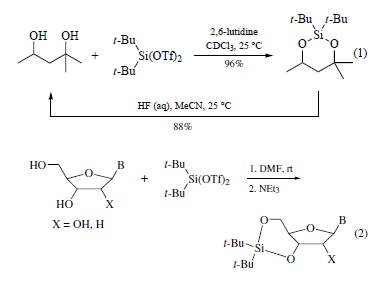
The Uses of DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE)
Protecting group reagent for 1,3-diols used recently in a synthesis of N-homoceramides. Also used for selective α-galactosylation in the synthesis of α-galactosyl ceramides.
Preparation
by the treatment of di-t-butylchlorosilane with trifluoromethanesulfonic acid, followed by distillation (71% yield).
Properties of DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE)
| Boiling point: | 73-75 °C/0.35 mmHg (lit.) |
| Density | 1.352 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 195 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | sol most common organic solvents. |
| form | Oil |
| color | Clear Pale Yellow |
| Specific Gravity | 1.358 |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 3569211 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 85272-31-7(CAS DataBase Reference) |
Safety information for DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE)
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE)
| InChIKey | HUHKPYLEVGCJTG-UHFFFAOYSA-N |
Abamectin manufacturer
JSK Chemicals
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 85272-31-7 Di-tert-butylsilylbis(trifluoromethanesul 99%View Details
85272-31-7 Di-tert-butylsilylbis(trifluoromethanesul 99%View Details
85272-31-7 -
 Di-tert-butylsilyl bis(trifluoromethanesulfonate) 95% CAS 85272-31-7View Details
Di-tert-butylsilyl bis(trifluoromethanesulfonate) 95% CAS 85272-31-7View Details
85272-31-7 -
 Di-tert-butylsilyl Bis(trifluoromethanesulfonate) CAS 85272-31-7View Details
Di-tert-butylsilyl Bis(trifluoromethanesulfonate) CAS 85272-31-7View Details
85272-31-7 -
 Di-tert-butylsilyl bis(trifluoromethanesulfonate) CAS 85272-31-7View Details
Di-tert-butylsilyl bis(trifluoromethanesulfonate) CAS 85272-31-7View Details
85272-31-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4