Dexpanthenol
Synonym(s):Dexpanthenol;Provitamin B;(R)-(+)-2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide;(R)-2,4-Dihydroxy-3,3-dimethylbutyric 3-hydroxypropylamide;D -Panthenol
- CAS NO.:81-13-0
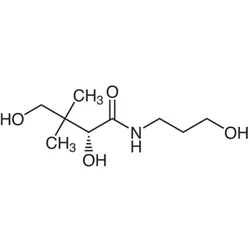
- Empirical Formula: C9H19NO4
- Molecular Weight: 205.25
- MDL number: MFCD00065006
- EINECS: 201-327-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-29 14:06:09
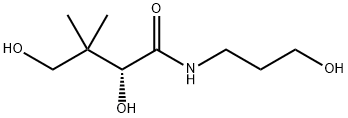
What is Dexpanthenol?
Absorption
Dexpanthenol is soluble in water and alcohol, although insoluble in fats and oil based substances. With the appropriate vehicle, Dexpanthenol is easily penetrated into the skin. Rate of penetration and absorption is reduced when Dexpanthenol is administered as an oil/water formula.
Toxicity
Mouse LD50 : 9gm/kg (Intraperitoneal) Mouse: LD50 7gm/kg (Intravenous) Mouse: LD50 15gm/kg (Oral) Rabbit LD50 4gm/kg (Oral)
Background
Dexpanthenol is a derivative of pantothenic acid, a B complex vitamin.
Dexpanthenol Injection is a sterile, nonpyrogenic, aqueous solution
indicated for use as a gastrointestinal stimulant.
Due to its good penetration and high local concentrations, dexpanthanol is used in many topical products, such as ointments and lotions for treatment of dermatological conditions to relieve itching or promote healing. Dermatological effects of the topical use of dexpanthenol include increased fibroblast proliferation and accelerated re-epithelialization in wound healing. Furthermore, it acts as a topical protectant, moisturizer, and has demonstrated anti-inflammatory properties .
Dexpanthenol is also available as a racemic mixture containing both the dextrorotatory form (dexpanthenol) and the levorotatory form (levopanthenol) as Panthenol. While pantothenic acid is optically active, only the dextrorotatory form (dexpanthenol) is biologically active.
Indications
Injection: Prophylactic use immediately after major abdominal surgery to minimize the possibility of paralytic ileus. Intestinal atony causing abdominal distention; postoperative or postpartum retention of flatus, or postoperative delay in resumption of intestinal motility; paralytic ileus.
Topical: This medication is used as a moisturizer to treat or prevent dry, rough, scaly, itchy skin and minor skin irritations (e.g., diaper rash, skin burns from radiation therapy).
Definition
ChEBI: Pantothenol is a monocarboxylic acid amide that is 3,3-dimethylbutanamide substituted by hydroxy groups at positions 2 and 4 and a 3-hydroxypropyl group at the carbomyl nitrogen. It has a role as a cholinergic drug and a provitamin. It is an amino alcohol and a monocarboxylic acid amide.
Pharmacokinetics
Pantothenic acid is a precursor of coenzyme A, which serves as a cofactor for a variety of enzyme-catalyzed reactions involving transfer of acetyl groups. The final step in the synthesis of acetylcholine consists of the choline acetylase transfer of acetyl group from acetylcoenzyme A to choline. Acetylcholine is the neurohumoral transmitter in the parasympathetic system and as such maintains the normal functions of the intestine. Decrease in acetylcholine content would result in decreased peristalsis and in extreme cases adynamic ileus.
Metabolism
Dexpanthenol is readily converted to pantothenic acid which is widely distributed into body tissues, mainly as coenzyme A.
The Uses of Dexpanthenol
Dexpanthenol is used as a moisturizer to treat or prevent dry, rough, scaly, itchy skin and minor skin irritations (such as diaper rash, skin burns from radiation therapy).
Properties of Dexpanthenol
| Melting point: | 64~69℃ |
| alpha | 30.5 º (c=5, H2O on anh. sub) |
| Boiling point: | 118-120 °C (2.7 mmHg) |
| Density | 1.20 g/mL at 20 °C (lit.) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.495-1.502 |
| Flash point: | 118-120°C/0.02m |
| storage temp. | 2-8°C |
| solubility | DMSO (Sparingly), Methanol (Slightly), Water (Sparingly) |
| form | Viscous Liquid or Semi Solid |
| pka | 13.03±0.20(Predicted) |
| color | Clear colorless to slightly yellow |
| PH | pH (100g/l,25℃) : 8.5~10.5 |
| optical activity | [α]20/D +30±1°, c = 5% in H2O |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,2947 |
| BRN | 1724947 |
| CAS DataBase Reference | 81-13-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Dexpanthenol(81-13-0) |
| EPA Substance Registry System | (+)-Panthenol (81-13-0) |
Safety information for Dexpanthenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dexpanthenol
| InChIKey | SNPLKNRPJHDVJA-ZETCQYMHSA-N |
Dexpanthenol manufacturer
Aethon International LLP
Unicorn Petroleum Industries Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![PANTOTHENIC ACID SODIUM SALT, D-, [2,3-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB8742328.gif)
You may like
-
 Dexpanthenol 95% CAS 81-13-0View Details
Dexpanthenol 95% CAS 81-13-0View Details
81-13-0 -
 D-Panthenol CAS 81-13-0View Details
D-Panthenol CAS 81-13-0View Details
81-13-0 -
 D-Panthenol 98.00% CAS 81-13-0View Details
D-Panthenol 98.00% CAS 81-13-0View Details
81-13-0 -
 D-Pantothanol CAS 81-13-0View Details
D-Pantothanol CAS 81-13-0View Details
81-13-0 -
 D PanthenolView Details
D PanthenolView Details
81-13-0 -
 D - Panthenol (75%), Grade: Unijell Dp 114View Details
D - Panthenol (75%), Grade: Unijell Dp 114View Details
81-13-0 -
 D-Panthenol (Pro Vitamin B5), Grade Standard: IP, Greater than 99%View Details
D-Panthenol (Pro Vitamin B5), Grade Standard: IP, Greater than 99%View Details
81-13-0 -
 99% D-Panthenol (working standard), Analytical GradeView Details
99% D-Panthenol (working standard), Analytical GradeView Details
81-13-0
