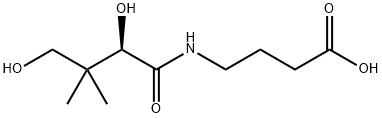
Panthenol
Synonym(s):(±)-α,γ-Dihydroxy-N-(3-hydroxypropyl)-β,β-dimethylbutyramide;(±)-2,4-Dihydroxy-3,3-dimethylbutyric 3-hydroxypropylamide;DL -Pantothenyl alcohol
- CAS NO.:16485-10-2
- Empirical Formula: C9H19NO4
- Molecular Weight: 205.25
- MDL number: MFCD00002944
- EINECS: 240-540-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
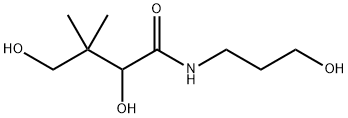
What is Panthenol?
The Uses of Panthenol
panthenol (pro-vitamin B5) acts as a penetrating moisturizer. Panthenol appears to stimulate cellular proliferation and aid in tissue repair. Studies indicate that when topically applied, panthenol penetrates the skin and gets converted into pantothenic acid, a B complex vitamin. Such action could possibly influence the skin’s natural resources of pantothenic acid. It imparts a nonirritant, non-sensitizing, moisturizing, and conditioning feel and promotes normal keratinization and wound-healing. Panthenol protects the skin against sunburn, provides relief for existing sunburn, and enhances the natural tanning process. Panthenol’s humectant character enables it to hold water in the product or attract water from the environment, resulting in a moisturizing effect. It enhances skin suppleness, and claims are that it also acts as an anti-inflammatory agent. It is considered a non-comedogenic raw material.
The Uses of Panthenol
vitamin B5 precursor, radioprotectant, antimalarial
The Uses of Panthenol
DL-Panthenol is used in the topical treatment of skin disorders, such as minor irritations and skin burns. Also used in the strengthening and treatment of hair.
Indications
Panthenol (containing a racemic mixture of dexpanthenol and levopanthenol) is not currently available in any FDA-approved products and therefore does not have an indication.
Please see Dexpanthenol for FDA-approved uses of the dextrorotatory form of Panthenol.
Background
Panthenol is an alcohol derivative of pantothenic acid, a component of the B complex vitamins and an essential component of a normally functioning epithelium. Panthenol exists as a racemic mixture containing both the dextrorotatory form (dexpanthenol) and the levorotatory form (levopanthenol). While pantothenic acid is optically active, only the dextrorotatory form (Dexpanthenol) is biologically active.
Dexpanthenol, the active form of panthenol, is enzymatically cleaved to form pantothenic acid (Vitamin B5), which is an essential component of Coenzyme A that acts as a cofactor in many enzymatic reactions that are important for protein metabolism in the epithelium .
Due to its good penetration and high local concentrations, dexpanthanol is used in many topical products, such as ointments and lotions for treatment of dermatological conditions to relieve itching or promote healing. Dermatological effects of the topical use of dexpanthenol include increased fibroblast proliferation and accelerated re-epithelialization in wound healing. Furthermore, it acts as a topical protectant, moisturizer, and has demonstrated anti-inflammatory properties .
General Description
Panthenol is an alcohol derivative of pantothenic acid, a component of the B complex vitamins and an essential component of a normally functioning epithelium. Panthenol exists in two optically active forms, both of which are used in pharmaceutical preparations. While pantothenic acid is optically active, only the dextrorotatory form is biologically active. Dexpanthenol, the active form of panthenol, is enzymatically cleaved to form pantothenic acid (Vitamin B5), which is an essential component of Coenzyme A that acts as a cofactor in many enzymatic reactions that are important for protein metabolism in the epithelium.
Flammability and Explosibility
Non flammable
Pharmacokinetics
Pantothenic acid is a precursor of coenzyme A, which serves as a cofactor for a variety of enzyme-catalyzed reactions involving transfer of acetyl groups. The final step in the synthesis of acetylcholine consists of the choline acetylase transfer of acetyl group from acetylcoenzyme A to choline. Acetylcholine is the neurohumoral transmitter in the parasympathetic system and as such maintains the normal functions of the intestine. Decrease in acetylcholine content would result in decreased peristalsis and in extreme cases adynamic ileus.
Metabolism
Properties of Panthenol
| Melting point: | 66-69 °C (lit.) |
| Boiling point: | 118-120 °C(Press: 0.02 Torr) |
| alpha | 0°(c=5, H2O) |
| Density | 1.166±0.06 g/cm3(Predicted) |
| vapor pressure | 0.004Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Acetonitrile (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 13.03±0.20(Predicted) |
| form | neat |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in water, ethanol, ether, chloroform, and propylene glycol. |
| Sensitive | Hygroscopic |
| Merck | 14,2947 |
| BRN | 1724945 |
| CAS DataBase Reference | 16485-10-2(CAS DataBase Reference) |
| EPA Substance Registry System | Butanamide, 2,4-dihydroxy-N-(3-hydroxypropyl)-3,3-dimethyl- (16485-10-2) |
Safety information for Panthenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Panthenol
| InChIKey | SNPLKNRPJHDVJA-UHFFFAOYSA-N |
Panthenol manufacturer
Dadia Chemical Industries
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 16485-10-2 dl-Panthenol 99%View Details
16485-10-2 dl-Panthenol 99%View Details
16485-10-2 -
 16485-10-2 98%View Details
16485-10-2 98%View Details
16485-10-2 -
 Dl-panthenol 98% CAS 16485-10-2View Details
Dl-panthenol 98% CAS 16485-10-2View Details
16485-10-2 -
 DL-Panthenol CAS 16485-10-2View Details
DL-Panthenol CAS 16485-10-2View Details
16485-10-2 -
 DL-Panthenol CAS 16485-10-2View Details
DL-Panthenol CAS 16485-10-2View Details
16485-10-2 -
 Panthenol, racemic CAS 16485-10-2View Details
Panthenol, racemic CAS 16485-10-2View Details
16485-10-2 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1