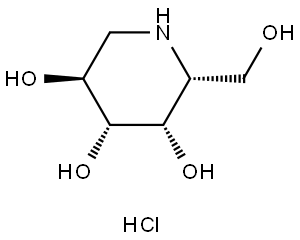
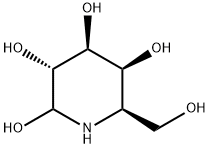
DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE
- CAS NO.:108147-54-2
- Empirical Formula: C6H13NO4
- Molecular Weight: 163.17
- MDL number: MFCD09910532
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
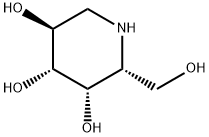
What is DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE?
Absorption
With absorption occurring largely in the gut, the absolute bioavailability (AUC) for a single oral 150 mg migalastat hydrochloride dose or a single 2-hour 150 mg intravenous infusion was approximately 75% and Tmax was approximately 3 hours. Plasma migalastat exposure (AUC0-∞) and Cmax demonstrated dose-proportional increases at migalastat hydrochloride oral doses from 50 mg to 1,250 mg (doses from 0.5 to 8.3-fold of the approved recommended dosage).
Migalastat administered with a high-fat meal (850 calories; 56% from fat), or 1 hour before a high-fat or light meal (507 calories; 30% from fat), or 1 hour after a light meal, resulted in significant reductions of 37% to 42% in mean total migalastat exposure (AUC0-∞) and reductions of 15% to 39% in mean peak migalastat exposure (Cmax) compared with the fasting state.
Toxicity
The most common adverse reactions reported with migalastat (≥ 10%) during the 6-month placebo-controlled, double-blind phase of its Study 1 clinical studies were headache, nasopharyngitis, urinary tract infection, nausea, and pyrexia .
In case of overdose, general medical care is recommended . Headache and dizziness were the most common adverse reactions reported at doses of migalastat of up to 1250 mg and 2000 mg, respectively .
Indications
Migalastat is approved by the FDA for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data.
This indication is approved under accelerated approval based on a reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Migalastat is also approved by the EMA and Health Canada to treat the same disease, although it is approved for both adults and adolescents aged 16 years and older in Europe.
Background
Fabry disease is a rare, progressive genetic disorder characterized by a defective GLA gene that causes a deficiency in the enzyme alpha-Galactosidase A (alpha-Gal A). This enzyme is responsible for breaking down glycosphingolipid substrate that, when deficient in patients with Fabry disease, builds up in the blood vessels, the kidneys, the nerves, the heart, and other organs. In the U.S., it is estimated that more than 3,000 people are living with Fabry disease, and an estimated more than 50 percent of these diagnosed patients are currently untreated.
Migalastat (approved and sold under Amicus Therapeutics' brand name Galafold) is subsequently an oral pharmacological chaperone of alpha-Gal A for the treatment of Fabry disease in adults who have amenable GLA variants. In these patients, migalastat works by stabilizing the body’s dysfunctional alpha-Gal A enzyme so that it can clear the accumulation of glycosphingolipid disease substrate. Globally, it is estimated that approximately 35 to 50 percent of Fabry patients may have amenable GLA variants that are treatable with migalastat.
Given the rarity of Fabry disease and the proportion of Fabry disease patients that could benefit from migalastat therapy, Amicus Therapeutics' brand name Galafold was approved using the Accelerated Approval pathway, under which the FDA may approve drugs for serious conditions where there is an unmet medical need and where a drug is shown to have certain effects that are reasonably likely to predict a clinical benefit to patients. A further study is required to verify and describe the clinical benefits of Galafold, and the sponsor will be conducting a confirmatory clinical trial of Galafold in adults with Fabry disease.
Additionally, Galafold was also granted Priority Review designation, under which the FDA’s goal is to take action on an application within six months of application filing where the agency determines that the drug if approved, would provide a significant improvement in treating, diagnosing or preventing a serious condition over available therapies. Galafold also received Orphan Drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
As of August 2018, migalastat under Amicus Therapeutics' brand name Galafold is currently approved in Australia, Canada, European Union, Israel, Japan, South Korea, Switzerland, and the United States.
Definition
ChEBI: Migalastat is a member of piperidines.
brand name
Treatment of Fabry disease.
Pharmacokinetics
In general, treatment in patients with migalastat in Phase 2 pharmacodynamic trials resulted in increases in endogenous alpha-galactosidase (alpha-Gal A) activity in white blood cells, as well as in skin and kidney for the majority of patients . In patients with amenable galactosidase alpha gene (GLA) mutations, globotriaosylceramide (GL-3) levels tended to decrease in the urine and in the kidney interstitial capillaries.
In an in vitro assay (HEK-293 assay), Human Embryonic Kidney (HEK-293) cell lines were transfected with specific GLA variants (mutations) which produced mutant alpha-Gal A proteins. In the transfected cells, amenability of the GLA variants was assessed after a 5-day incubation with 10 micromol/L migalastat. A GLA variant was categorized as amenable if the resultant mutant alpha-Gal A activity (measured in the cell lysates) met two criteria: 1) it showed a relative increase of at least 20% compared to the pre-treatment alpha-Gal A activity, and 2) it showed an absolute increase of at least 3% of the wild-type (normal) alpha-Gal activity. The in vitro assay did not evaluate the trafficking of the mutant alpha-Gal A proteins into the lysosome or the dissociation of migalastat from the mutant alpha-Gal A proteins within the lysosome. Also, the in vitro assay did not test whether a GLA variant causes Fabry disease or not.
Metabolism
Based upon in vivo data, migalastat is a substrate for uridine diphosphate glucuronosyltransferase (otherwise known as UGT or UDPGT), being a minor elimination pathway.
Properties of DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE
| Melting point: | 243-245 °C |
| Boiling point: | 361.1±42.0 °C(Predicted) |
| Density | 1.456±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| pka | 13.77±0.70(Predicted) |
Safety information for DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE
Computed Descriptors for DEOXYGALACTONOJIRIMYCIN, HYDROCHLORIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8