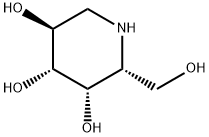
Deoxygalactonojirimycin Hydrochloride
Synonym(s):1,5-Dideoxy-1,5-imino-D -galactitol;Deoxygalactonojirimycin, Hydrochloride - CAS 75172-81-5 - Calbiochem;DGJ, 1,5-Dideoxy-1,5-imino-D-galactitol, Galactostatin, HCl;Migalastat
- CAS NO.:75172-81-5
- Empirical Formula: C6H13NO4.ClH
- Molecular Weight: 199.63
- MDL number: MFCD00269962
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-13 21:11:14
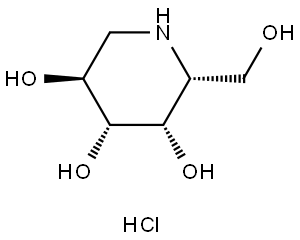
What is Deoxygalactonojirimycin Hydrochloride?
Description
Migalastat, which is marketed by Amicus Therapeutics, received approval in the EU for the treatment of Fabry disease in adults and adolescents aged 16 or older. Fabry disease is caused by mutations of the enzyme α-galactosidase A (α-GAL A) that cause protein misfolding and prevents efficient metabolism of the glycosphingolipid globotriaosylceramide (GL3). Accumulation of GL3 in lysosomes, blood vessels, and various tissues ultimately leads to significant heart, kidney, and dermatological problems. Migalastat functions as a molecular chaperone to α- GAL A, engaging the enzyme and enabling it to adopt the proper conformation allowing for efficient breakdown of GL3. Because the standard of care prior to 2016 for treating Fabry disease was enzyme replacement therapy (ERT), migalastat’s approval in the EU represents an important advance for patients suffering from this disorder.
Chemical properties
White Crystalline Solid
The Uses of Deoxygalactonojirimycin Hydrochloride
Proven to be an extremely potent and selective a-D-galactosidase inhibitor.
The Uses of Deoxygalactonojirimycin Hydrochloride
inhibitor of b-glucosidase
The Uses of Deoxygalactonojirimycin Hydrochloride
Deoxygalactonojirimycin hydrochloride has been used as an α-galactosidase A inhibitor to assess the enzymatic activity of α-galactosidase A. It has also been used as an α-galactosidase A inhibitor to study its effects on the mRNA levels in human embryonic kidney (HEK) cells and hippocampal neurons.
What are the applications of Application
Deoxygalactonojirimycin Hydrochloride is a potent and selective a-galactosidase inhibitor
brand name
Treatment of Fabry disease.
Biochem/physiol Actions
Deoxygalactonojirimycin hydrochloride is an inhibitor of α-galactosidase A. Deoxygalactonojirimycin exhibits therapeutic effects against Fabry disease.
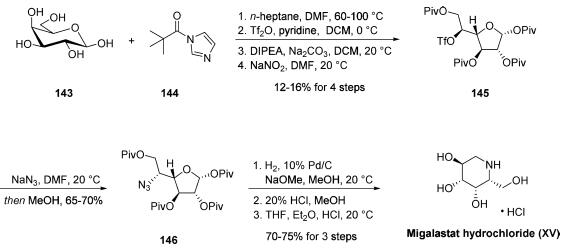
Synthesis
Several unique synthetic approaches to migalastat, which is
also known as D-1-deoxygalactonojirimycin (DGJ), have been
reported in the literature. Although the most likely
commercial-scale preparation of this drug proceeds through a
microbial fermentation process disclosed in a 2015 patent, a
kilogram-scale synthesis of the drug outlined has
been described in a 2008 patent application filed by Amicus.
This route closely resembles a procedure disclosed in 1999 by
Uriel and Santoyo-Gonzalez that presented handling and
safety concerns. Commercial D-galactose (143) was treated
with five equivalents of pivaloyl imidazole (144), followed by
triflation, treatment with Hunig?ˉs base, and exposure to sodium
nitrite to furnish the tetrapivaloyl altofuranose triflate 145 after
recrystallization from heptane. Next, stereospecific azide
displacement of the triflate successfully delivered azidofuranose 146 in 65-70% yield. This reaction generated over 3 kg of the
desired alkyl azide after recrystallization from ethanol and
water. Lastly, palladium-catalyzed hydrogenolysis in the
presence of sodium methoxide, a methanolic acidification
step, and then a subsequent acidification step using HCl in
THF furnished migalastat hydrochloride (XV) in 70-75% yield
over the three-step sequence from 146.
References
[1] asano n, ishii s, kizu h, et al. in vitro inhibition and intracellular enhancement of lysosomal α‐galactosidase a activity in fabry lymphoblasts by 1‐deoxygalactonojirimycin and its derivatives[j]. febs journal, 2000, 267(13): 4179-4186.
[2] ishii s, chang h, yoshioka h, et al. preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for fabry disease[j]. journal of pharmacology and experimental therapeutics, 2009, 328(3): 723-731.
Properties of Deoxygalactonojirimycin Hydrochloride
| Melting point: | 260 |
| storage temp. | 2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | White crystalline solid |
| color | White to Brown |
Safety information for Deoxygalactonojirimycin Hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Deoxygalactonojirimycin Hydrochloride
Deoxygalactonojirimycin Hydrochloride manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![D-GALACTOSE 1-[2-(2-AZIDOETHOXY)ETHOXYETHYL]-2,3,4,6-TETRA-O-ACETATE](https://img.chemicalbook.in/CAS/GIF/381716-33-2.gif)
You may like
-
 Migalastat Hydrochloride 98%View Details
Migalastat Hydrochloride 98%View Details -
 Deoxygalactonojirimycin hydrochloride CAS 75172-81-5View Details
Deoxygalactonojirimycin hydrochloride CAS 75172-81-5View Details
75172-81-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1