DBDCB
Synonym(s):1,2-Dibromo-2,4-dicyanobutane;1-Bromo-1-(bromomethyl)-1,3-propanedicarbonitrile;2-Bromo-2-(bromomethyl)glutaronitrile
- CAS NO.:35691-65-7
- Empirical Formula: C6H6Br2N2
- Molecular Weight: 265.93
- MDL number: MFCD00072483
- EINECS: 252-681-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-24 17:31:20
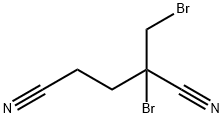
What is DBDCB?
Description
1,2-Dibromo-2,4-dicyanobutane is the main sensitizer of Euxyl K400, a widely used preservative agent in cosmetics, toiletries or metalwork fluids.
Methyldibromo glutaronitrile (MDBGN), also known as dibromocyanobutane, is to be found in the preservative system Euxyl K 400 (also called Tektamer 38), which is a mix of MDBGN and phenoxyethanol in a ratio of 1:4. Euxyl K 400 is a broad‐spectrum preservative with activity against fungi and bacteria. MDBGN is nearly always the allergen when sensitization to Euxyl K 400 occurs.
Chemical properties
Light yellow crystalline solid or powder. Commercial products may be soluble concentrate (liquid or solid); pellets/tablets. Mild, acrid, sweet odor.
Occurrence
MDBGN was historically widely used in cosmetics, sunscreens, shampoos, liquid soaps, and barrier and moisturizing creams used at work. Other sources include moistened toilet tissues, ultrasound gel, adhesives, soluble cutting oils, latex paints, vaginal examination gel and sanitary pads. The EU ban has reduced these exposures very signifi cantly. Nevertheless, its inclusion in products used in the workplace such as barrier creams and after‐work creams makes its inclusion in the European and BSCA baseline series still relevant at the current time.
The Uses of DBDCB
1,2-Dibromo-2,4-dicyanobutane is a preservative for metalworking fluids, cosmetics, adhesives, latex emulsions and paints, dispersed pigments and detergents; active ingredient in Euxyl K 400 and Tektamer 38.
Background
Bromothalonil, also known as Methyldibromo glutaronitrile (MDBGN), is a widely used preservative that can be found in many personal hygiene and industrial products. It is also a known allergen and dermatological irritant that has been banned in many EU countries due to increasing rates of contact allergy . In 2005, MDBGN was named in the top 15 most frequently positive allergens identified in patch tests by the North American Contact Dermatitis Group (NACDG).
Sensitivity to Bromothalonil may be identified with a clinical patch test.
Indications
Bromothalonil is approved for use within allergenic epicutaneous patch tests which are indicated for use as an aid in the diagnosis of allergic contact dermatitis (ACD) in persons 6 years of age and older.
Definition
ChEBI: An organobromine compound that consists of pentanedinitrile bearing bromo and bromomethyl substituents at position 2.
General Description
Crystals with a pungent odor. Insoluble in water. Used as a preservative in latex paint, adhesives, etc.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
DBDCB may polymerize in the presence of metals and some metal compounds. Incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents.
Agricultural Uses
Microbiocide: Tolerances have been established by the U.S. Food and Drug Administration when this substances is used as a preservative in food-grade adhesives and as a slimicide in the manufacture of food-grade paper and paperboard. Used to control slime-forming bacteria and fungi in recirculating water cooling system; oil-recovery drilling mud systems; paper mill and pulp mill water systems and similar industrial processing and chemical systems.
Trade name
BIOCHEK®; BIOCLEAR®; MERCK® 48051; METACIDE® 38; METASOL; TEKTAMER
Contact allergens
Methyldibromo glutaronitrile is a biocide widely used as a preservative agent in cosmetics, toiletries, and metalworking fluids. It is a potent allergen, banned in all cosmetics in the EU since 2007.
Safety Profile
Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NO, and Br-.
Potential Exposure
Nitrile microbiocide used as a preser- vative in food grade adhesives and as a slimicide in the manufacture of food grade paper and paperboard; used to control slime-forming bacteria and fungi in recirculating water cooling system; oil recovery drilling mud systems; paper mill and pulp mill water systems and similar indus- trial processing and chemical systems.
Metabolism
Not Available
Shipping
UN3261 Corrosive solid, acidic, organic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN3439 Nitriles, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Corrosive. Strong oxidizers and reducing agents, strong acids and bases. Reacts with acids, steam, warm water producing toxic and flammable hydrogen cya- nide fumes. Hydrogen cyanide is produced when propioni- trile is heated to decomposition. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give car- boxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids .
Waste Disposal
Recycle any unused portion of the material for its approved use or return it to the manu- facturer or supplier. Ultimate disposal of the chemical must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations .
Properties of DBDCB
| Melting point: | 48-50°C |
| Boiling point: | 338.6±42.0 °C(Predicted) |
| Density | 1.9436 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| storage temp. | 2-8°C |
| Water Solubility | Insoluble in water |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| color | White to Almost white |
| Merck | 14,3021 |
| BRN | 1930600 |
| CAS DataBase Reference | 35691-65-7(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Bromo-1-(bromomethyl)-1,3-propanedicarbonitrile (35691-65-7) |
Safety information for DBDCB
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DBDCB
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 2-Bromo-2-(bromomethyl)glutaronitrile CAS 35691-65-7View Details
2-Bromo-2-(bromomethyl)glutaronitrile CAS 35691-65-7View Details
35691-65-7 -
 Methyldibromoglutaronitrile CAS 35691-65-7View Details
Methyldibromoglutaronitrile CAS 35691-65-7View Details
35691-65-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4