CYTOCHALASIN J
- CAS NO.:53760-20-6
- Empirical Formula: C28H37NO4
- Molecular Weight: 451.6
- MDL number: MFCD00077708
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
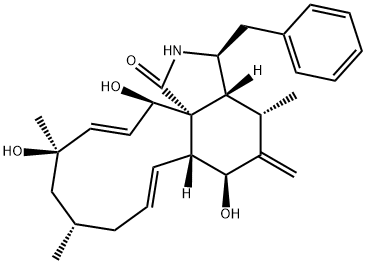
What is CYTOCHALASIN J?
The Uses of CYTOCHALASIN J
Cytochalasin J is a cell-permeable fungal toxin used in actin polymerization studies and cytological research.
What are the applications of Application
Cytochalasin J is a cell-permeable fungal toxin used in actin polymerization studies and cytological research
Biological Activity
cytochalasin j can alter mitotic spindle microtubule organization and kinetochore structure.the cytochalasins are cell-permeable fungal metabolites inhibiting actin polymerization, which can alter diverse processes including cell movement, growth, phagocytosis, degranulation, and secretion.
in vitro
cytochalasin j was identified as a deacetylated analog of cytochalasin h that weakly inhibited actin assembly but significantly altered mitotic spindle microtubule organization and kinetochore structure [1]. in a previous study, cytochalasin j treatment of prometaphase cells reduced the number of kmts and the size and organization of the kinetochore lamina. moreover, in approximately 30% of cells treated with cytochalasin j, the failure of a small number of chromosomes to attach to spindle fibers could be documented. these chromosomes showed a significant change in the organization of the kinetochore laminae. light microscopic analyses of cells treated with cytochalasin j revealed loss of chromosome congression, with chromsomes usually located at the periphery of the spindle. cells treated with 10 microg/ml cytochalasin j for 10 min and released into tissue culture medium showed a resumption of chromosome motion within a few minutes, both during congression and anaphase [2].
storage
+4°C
References
[1] walling, e. a.,krafft, g.a. and ware, b.r. actin assembly activity of cytochalasins and cytochalasin analogs assayed using fluorescence photobleaching recovery. archives of biochemistry and biophysics 264(1), 321-332 (1988).
[2] wrench, g. a. and snyder, j.a. cytochalasin j treatment significantly alters mitotic spindle microtubule organization and kinetochore structure in ptk1 cells. cell motility and the cytoskeleton 36(2), 112-124 (1997).
Properties of CYTOCHALASIN J
| Melting point: | 138°C (dec.) |
| Boiling point: | 679.5±55.0 °C(Predicted) |
| Density | 1.20±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol;Soluble in methanol;Soluble in DMSO;Soluble in dimethyl formamide |
| form | white lyophilisate |
| pka | 13.03±0.70(Predicted) |
| color | White |
| Stability: | Light Sensitive |
Safety information for CYTOCHALASIN J
Computed Descriptors for CYTOCHALASIN J
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





![[4(N)-3H]CYTOCHALASIN B,CYTOCHALASIN B, [4-3H(N)]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6368649.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1