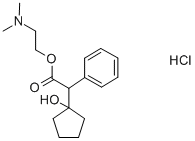
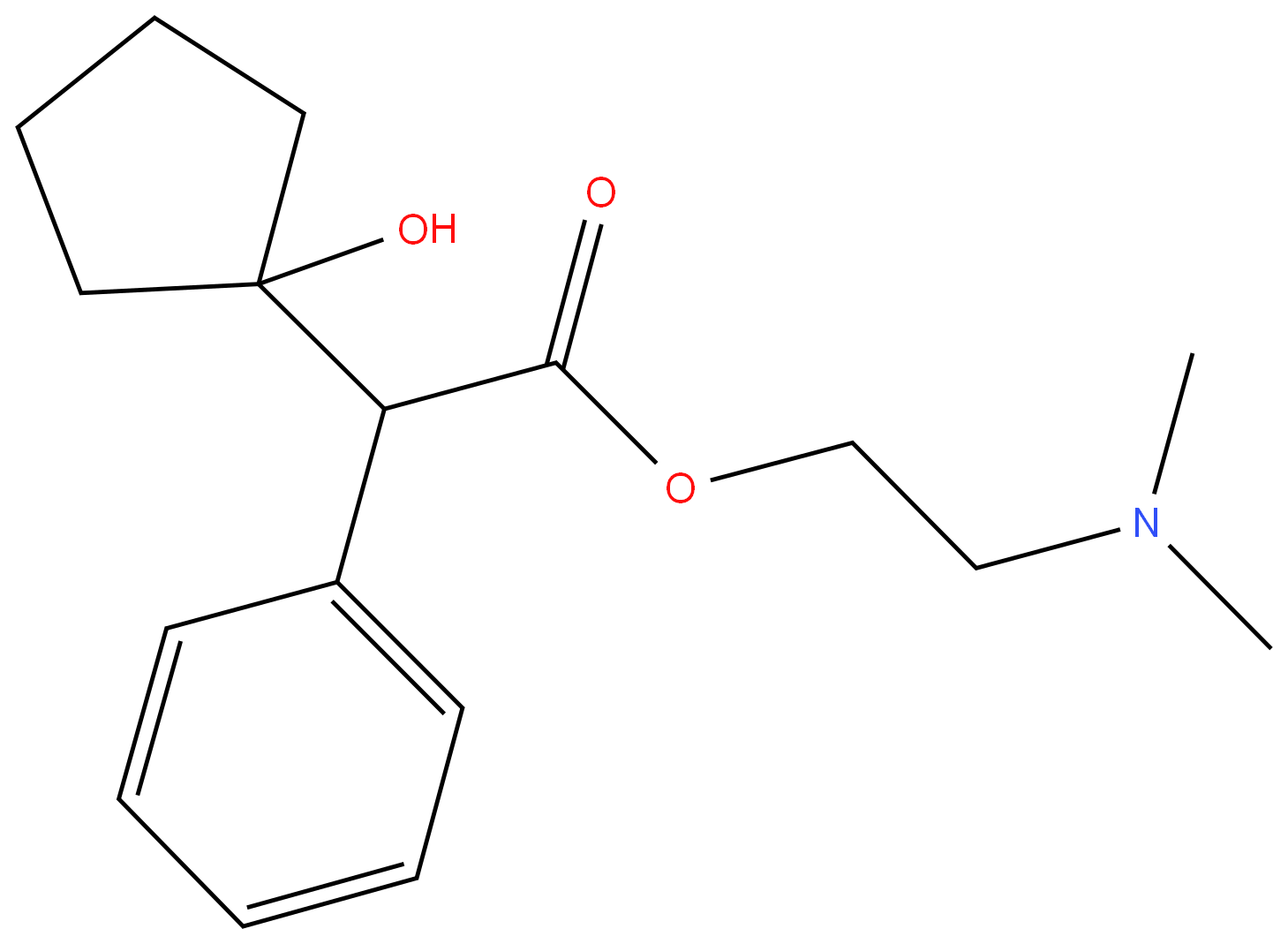
CYCLOPENTOLATE
- CAS NO.:512-15-2
- Empirical Formula: C17H25NO3
- Molecular Weight: 291.39
- MDL number: MFCD00599448
- EINECS: 208-136-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
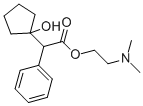
What is CYCLOPENTOLATE?
Absorption
Absorbed following ophthalmic administration.
Toxicity
Oral LD50 in the rat is 4000 mg/kg and 960 mg/kg in the mouse. Symptoms of overdose include tachycardia, dizziness, dry mouth, behavioral disturbances, uncoordination and drowsiness.
Originator
Cyclogyl,Schieffelin,US,1953
The Uses of CYCLOPENTOLATE
Cyclopentolate is an effective mydriatic and cycloplegic that begins to act very quickly and has a relatively short duration. It is used also in ophthalmoscopy for causing pre-operational mydriasis.
The Uses of CYCLOPENTOLATE
thalidomide derivative for multiple myeloma and myelodysplastic syndromes
The Uses of CYCLOPENTOLATE
Cyclopentolate is a muscarinic antagonist. It is used in eye drops to dilate the eyes and prevent them from refocusing making the eyes more sensitive to light and may cause blurring.
Background
A parasympatholytic anticholinergic used solely to obtain mydriasis or cycloplegia.
Indications
Used mainly to produce mydriasis and cycloplegia for diagnostic purposes.
Definition
ChEBI: A carboxylic ester resulting from the formal condensation of (1-hydroxycyclopentyl)(phenyl)acetic acid with N,N-dimethylethanolamine. A tertiary amine antimuscarinic with actions similar to atropine, it is used as its ydrochloride salt to produce mydriasis (excessive dilation of the pupil) and cycloplegia (paralysis of the ciliary muscle of the eye) for opthalmic diagnostic procedures. It acts more quickly than atropine and has a shorter duration of action.
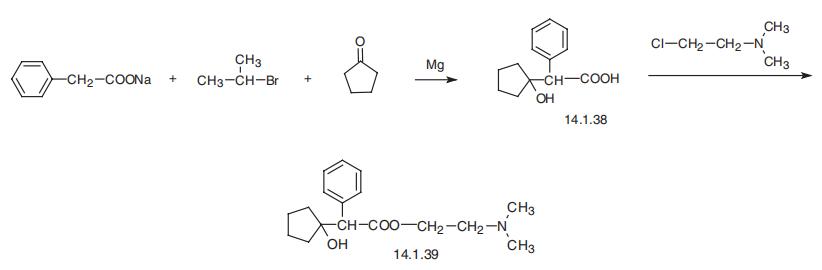
Manufacturing Process
To a well stirred suspension of 9 g of sodium phenyl acetate and 2.4 g of
magnesium turnings in 25 cc of anhydrous ether, a solution of 9.4 cc of
isopropyl bromide in 50 cc of anhydrous ether are added. The mixture is
refluxed for one hour (during which time propane is evolved) and then 5 cc of
cyclopentanone in 25 cc of anhydrous ether are added dropwise. The mixture
is then refluxed for one hour and poured over ice water containing some
hydrochloric acid. The ether solution is separated and extracted with 200 cc of
5% sodium hydroxide. The alkaline solution on acidification gives the free acid which is filtered off, dried in a desiccator and recrystallized from a mixture of
ethylene dichloride and petroleum ether.
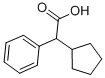
The product is 2-phenyl-2-(1-hydroxycyclopentyl)ethanoic acid, melting at 95°
to 97°C. Of this product, 4.5 g in 30 cc of dry isopropyl alcohol are refluxed
for 16 hours with 2.5 g of β-chloroethyl dimethyl amine. The solution is cooled
and filtered clear from the solid by-product. The solvent is removed under
reduced pressure on the steam bath and the residue is washed with
anhydrous ether. It is dissolved in ethyl acetate from which it crystallizes. It is
the hydrochloride of β-(dimethylamino)ethyl ester of 2-phenyl-2-(1-
hydroxycyclopentyl) ethanoic acid, melting at 134° to 136°C.
brand name
Akpentolate (Akorn); Cyclogyl (Alcon); Pentolair (Bausch & Lomb); Pentolair (Pharmafair).
Therapeutic Function
Anticholinergic (ophthalmic)
Pharmacokinetics
Cyclopentolate is an anti-muscarinic in the same class as atropine and scopolamine. Cyclopentolate blocks the receptors in the muscles of the eye (muscarinic receptors). These receptors are involved controlling the pupil size and the shape of the lens. Cyclopentolate thus induces relaxation of the sphincter of the iris and the ciliary muscles. When applied topically to the eyes, it causes a rapid, intense cycloplegic and mydriatic effect that is maximal in 15 to 60 minutes; recovery usually occurs within 24 hours. The cycloplegic and mydriatic effects are slower in onset and longer in duration in patients who have dark pigmented irises.
Synthesis
Cyclopentolate, 2-(dimethylamino)ethylic ester of 1-hydroxycyclopentane- |á-phenylacetic acid (14.1.39), is synthesized by the esterification of |á-(1- droxycylopentyl) phenylacetic acid (14.1.38) using 2-dimethylaminoethylchloride, |á-(1- Hydroxycyclopentyl) phenylacetic acid (14.1.38) is synthesized by reacting the sodium salt of phenylacetic acid with cyclopentanone in the presence of isopropylmagnesium bromide [30].
Veterinary Drugs and Treatments
Cyclopentolate is an anticholinergic agent that induces relaxation of the sphincter of the iris and the ciliary muscles. When applied topically to the eyes, it causes a rapid, intense cycloplegic and mydriatic effect that is maximal in 15 – 60 minutes; recovery usually occurs within 24 hours. The cycloplegic and mydriatic effects are slower in onset and longer in duration in animal patients who have darkly pigmented irises. Cyclopentolate is used mainly to produce mydriasis and cycloplegia for diagnostic purposes.
Metabolism
Not Available
Properties of CYCLOPENTOLATE
| Boiling point: | 409.7±20.0 °C(Predicted) |
| Density | 1.136±0.06 g/cm3(Predicted) |
| pka | pKa 7.93(H2O ) (Uncertain) |
Safety information for CYCLOPENTOLATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CYCLOPENTOLATE
CYCLOPENTOLATE manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 512-15-2 Cyclopentolate Hydrochloride 99%View Details
512-15-2 Cyclopentolate Hydrochloride 99%View Details
512-15-2 -
 Cyclopentolate 98%View Details
Cyclopentolate 98%View Details -
 Cyclopentolate 99%View Details
Cyclopentolate 99%View Details
512-15-2 -
 Cyclopentolate 95% CAS 512-15-2View Details
Cyclopentolate 95% CAS 512-15-2View Details
512-15-2 -
 Cyclopentolate 98% (HPLC) CAS 512-15-2View Details
Cyclopentolate 98% (HPLC) CAS 512-15-2View Details
512-15-2 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
