Cupric oxalate
- CAS NO.:814-91-5
- Empirical Formula: C2CuO4
- Molecular Weight: 151.56
- MDL number: MFCD00036413
- EINECS: 212-411-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
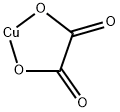
What is Cupric oxalate?
Description
Cupric oxalate is a bluish-white, odorless powder. Molecular weight=153.58. Hazard Identification(based on NFPA-704 M Rating System): Health 1,Flammability 0, Reactivity 1. Insoluble in water
Chemical properties
Cupric oxalate is a bluish-white, odorless powder.
The Uses of Cupric oxalate
As catalyst for organic reactions; as stabilizer for acetylated polyformaldehyde; in anticaries compositions; in seed treatments to repel birds and rodents.
Preparation
Copper(II) oxalate can be prepared by reaction of sodium oxalate with copper(II) salt solutions. Copper(II) oxalate is used as a catalyst in organic reactions and as a stabilizer for acetylated polyformaldehyde.
General Description
Odorless bluish-white solid. Denser than water and insoluble in water. Hence sinks in water. Used as a catalysts for organic reactions.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Cupric oxalate dissolves in aqueous ammonia and reacts as an acid to neutralize other bases as well. Can serve as a reducing agent in reactions that generate carbon dioxide.
Hazard
Toxic by ingestion; tissue irritant.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion of very large amounts may produce symptoms of oxalate poisoning; watch for edema of the glottis and delayed constriction of esophagus. Contact with eyes causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic carbon monoxide gas may form in fire.
Potential Exposure
Used as a catalyst for organic reactions and in seed treatment as a repellent for birds and rodents.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
storage
Color Code—Green: General storage may be used.Prior to working with cupric oxalate you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from acetylene gas, ammonia, caustic solutions, and nitromethane.
Shipping
UN2775, Copper based pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Explosive materials are formed on contact with acetylene gas, ammonia, caustic solutions; sodium hypobromite, nitromethane. Slight heating can cause a weak explosion. Cupric oxalate dissolves in aqueous ammonia and reacts as an acid to neutralize other bases as well. Can serve as a reducing agent in reactions that generate carbon dioxide. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Properties of Cupric oxalate
| Melting point: | anhydrous decomposes at ~300 to copper oxide [HAW93] |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | Aqueous Base (Slightly) |
| form | Powder |
| color | blue |
| Water Solubility | insoluble |
| Solubility Product Constant (Ksp) | pKsp: 9.35 |
| CAS DataBase Reference | 814-91-5(CAS DataBase Reference) |
| EPA Substance Registry System | Cupric oxalate (814-91-5) |
Safety information for Cupric oxalate
Computed Descriptors for Cupric oxalate
Cupric oxalate manufacturer
New Alliance Dye Chem Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
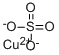
You may like
-
 814-91-5 Cupric oxalate 98%View Details
814-91-5 Cupric oxalate 98%View Details
814-91-5 -
 Cupric oxalate CAS 814-91-5View Details
Cupric oxalate CAS 814-91-5View Details
814-91-5 -
 Copper Oxalate CASView Details
Copper Oxalate CASView Details -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0