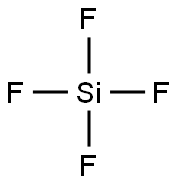Cryptohalite ((NH4)2(SiF6))
- CAS NO.:1309-32-6
- Empirical Formula: F6H4NSi-
- Molecular Weight: 160.11
- Update Date: 2024-12-18 14:15:30
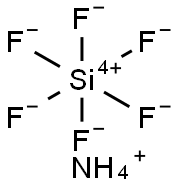
What is Cryptohalite ((NH4)2(SiF6))?
Chemical properties
Ammonium hexafluorosilicate is a white crystalline powder. Odorless.
Safety Profile
Poison by ingestion and subcutaneous routes. See also diHYDROGEN and FLUORIDES. When heated to decomposition it emits very toxic fumes of F-, NH3,
Potential Exposure
This material is used as a pesticide and miticide, wood preservative, soldering flux; light metal casting; and in the etching of glass.
Shipping
UN2856 Fluorosilicates, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Aqueous solution is highly corrosive. Contact with acids reacts to form hydrogen fluoride, which is a highly corrosive and forms a toxic gas. Corrosive to aluminum. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Ammonium fluorosilicate reacts with water to form hydrofluoric acid, a source of fluoride ions. Unlike other halide ions, the fluoride ion is highly reactive, acting as a weak base and participating in some unique reactions. In particular, fluorides react strongly with compounds containing calcium, magnesium, or silicon ions, which means that solutions containing soluble fluorides are corrosive to both living tissue and glass. Hydrofluoric acid can cause severe chemical burns and is one of the few materials that can etch glass. It is also a toxic gas in its anhydrous form.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration.
Properties of Cryptohalite ((NH4)2(SiF6))
| Density | 2.004 g/cm3(Temp: 25 °C) |
| EPA Substance Registry System | Cryptohalite ((NH4)2(SiF6)) (1309-32-6) |
Safety information for Cryptohalite ((NH4)2(SiF6))
Computed Descriptors for Cryptohalite ((NH4)2(SiF6))
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1