Creatine
Synonym(s):(α-Methylguanido)acetic acid;N-Amidinosarcosine;Creatine monohydrate
- CAS NO.:57-00-1
- Empirical Formula: C4H9N3O2
- Molecular Weight: 131.13
- MDL number: MFCD00004282
- EINECS: 200-306-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:40
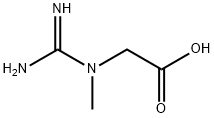
What is Creatine?
Chemical properties
White powder
Occurrence
Creatine is an amino acid that occurs naturally in dairy products, seafood, and beef. It is manufactured by the body in the liver, kidney, and pancreas.
The Uses of Creatine
Creatine is used for gyrate atrophy, McArdle's disease, muscular dystrophy, amyotrophic lateral sclerosis, and rheumatoid arthritis. It is used in congestive heart disease to improve exercise tolerance and to enhance athletic performance.
The Uses of Creatine
Creatine is involved in the rapid production of ATP associated with creatine kinases in skeletal muscle tissue. Creatine may be used as a supplement to study its mechanism of action. It acts as a nutraceutical and a neuroprotective agent.
Background
An amino acid derivative that occurs in vertebrate tissues and in urine. In muscle tissue, creatine generally occurs as phosphocreatine. Creatine is excreted as creatinine in the urine.
Indications
For nutritional supplementation, also for treating dietary shortage or imbalance.
What are the applications of Application
Creatine, anhydrous is involved in the rapid production of ATP in skeletal muscle tissue
Definition
ChEBI: Creatine is a glycine derivative having methyl and amidino groups attached to the nitrogen. It has a role as a neuroprotective agent, a nutraceutical, a human metabolite, a mouse metabolite and a geroprotector. It is a member of guanidines and a glycine derivative. It is a conjugate acid of a creatinate. It is a tautomer of a creatine zwitterion.
Biochem/physiol Actions
Creatine is a nitrogenous compound that acts as a high-energy reservoir for the rapid regeneration of ATP. Approximately 95% of creatine is found in skeletal muscle, primarily as phosphocreatine. Creatine can be acquired through dietary consumption or formed from L-arginine, glycine, and L-methionine in a multi-step reaction that occurs in the kidneys and liver. Creatine is then transported to muscle tissue. Creatine supplementation is used for the enhancement of sports performance, primarily by increasing muscle mass. Creatine is also being investigated as a treatment of neuromuscular diseases, where it may aid in neuroprotection and by improving the cellular bioenergetic state.
Pharmacokinetics
Creatine is a essential, non-proteinaceous amino acid derivative found in all animals. It is synthesized in the kidney, liver, and pancreas from L-arginine, glycine and L-methionine. Following its biosynthesis, creatine is transported to the skeletal muscle, heart, brain and other tissues. Most of the creatine is metabolized in these tissues to phosphocreatine (creatine phosphate). Phosphocreatine is a major energy storage form in the body. Supplemental creatine may have an energy-generating action during anaerobic exercise and may also have neuroprotective and cardioprotective actions.
Metabolism
Not Available
Purification Methods
Likely impurities are creatinine and other guanidino compounds. It crystallises from the minimum volume of boiling H2O as the monohydrate. The hydrate is also obtained by dissolving in H2O and adding Me2CO. Drying under vacuum over P2O5 or drying at 100o gives the anhydrous base. The anhydrous base can be obtained also by dissolving the hydrate in H2O, seeding with the anhydrous base and cooling in ice. A m of 258 -268o(dec) was reported. The picrate crystallises from 17 parts of H2O with m of 218-220o(dec). [King J Chem Soc 2377 1930, Hoffmann et al. J Am Chem Soc 58 1730 1936, Mendel & Hodgkin Acta Cryst 7 443 1954, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2750 1961, Beilstein 4 III 1170, 4 IV 2425.]
Properties of Creatine
| Melting point: | ~295 °C (dec.) |
| Boiling point: | 242.43°C (rough estimate) |
| Density | 1,33 g/cm3 |
| refractive index | 1.5700 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Water (Slightly, Heated) |
| form | Solid |
| pka | 2.63(at 25℃) |
| color | White to Off-White |
| Water Solubility | Soluble in water. |
| Merck | 14,2568 |
| BRN | 907175 |
| CAS DataBase Reference | 57-00-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Glycine, N-(aminoiminomethyl)-N-methyl-(57-00-1) |
| EPA Substance Registry System | Glycine, N-(aminoiminomethyl)-N-methyl- (57-00-1) |
Safety information for Creatine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Creatine
| InChIKey | CVSVTCORWBXHQV-UHFFFAOYSA-N |
Creatine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran







You may like
-
 57-00-1 Creatine, 98% 99%View Details
57-00-1 Creatine, 98% 99%View Details
57-00-1 -
 Creatine 98% CAS 57-00-1View Details
Creatine 98% CAS 57-00-1View Details
57-00-1 -
 Creatine CAS 57-00-1View Details
Creatine CAS 57-00-1View Details
57-00-1 -
 Creatine CAS 57-00-1View Details
Creatine CAS 57-00-1View Details
57-00-1 -
 CreatineView Details
CreatineView Details
57-00-1 -
 99% Creatine, Analytical GradeView Details
99% Creatine, Analytical GradeView Details
57-00-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
