COPPER(II) SULFIDE
Synonym(s):Copper Blue;Copper monosulfide;Cupric sulfide;Horace Vernet′s Blue
- CAS NO.:1317-40-4
- Empirical Formula: CuS
- Molecular Weight: 95.61
- MDL number: MFCD00016066
- EINECS: 215-271-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
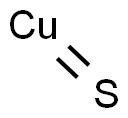
What is COPPER(II) SULFIDE?
Chemical properties
Copper(II) sulfide, CuS, [1317-40-4], MW 95.6, occurs in nature as the blue-black mineral covellite, [19138-68-2]. It is insoluble in water but is decomposed by nitric acid. In moist air, it is oxidized to copper(II) sulfate. The rate of oxidation is accelerated in the presence of alkali. Copper(II) sulfide is used as an antifouling pigment and in the preparation of aniline black dyes.
The Uses of COPPER(II) SULFIDE
Copper(II) sulfide is used in antifouling paints, mixed catalysts and to develop aniline black dyes in textile printing. Further, it is used as a varnish pigment and an oxidizing agent. In addition, it is used as a component of solid state electrodes and thin-film solar cell with cadmium sulfide. It is a moderate conductor of electricity and used in photovoltaics.
The Uses of COPPER(II) SULFIDE
In antifouling paints; in preparation of mixed catalysts; in development of aniline black dye in textile printing.
Definition
ChEBI: Copper(II) sulfide is a copper sulfide in which the metal is in the +2 oxidation state.
Preparation
Copper(II) sulfide is prepared by heating copper(I) sulfide in the presence of excess sulfur in a hydrogen atmosphere. Ethanol solutions of anhydrous copper(II) chloride precipitate the copper(II) sulfide on the addition of hydrogen sulfide.
General Description
Cu63 NMR, X ray photoelectron spectroscopy,2 copper sulfide CuS was studied in detail.
Hazard
Toxic by ingestion.
Properties of COPPER(II) SULFIDE
| Melting point: | 220 °C (dec.) (lit.) |
| Density | 4.6 g/mL at 25 °C (lit.) |
| refractive index | 1.45 |
| solubility | insoluble in H2O, ethanol, dilacid, alkaline solutions |
| form | powder |
| color | Black |
| Specific Gravity | 4.6 (20℃) |
| Water Solubility | Soluble in nitric acid, ammonium hydroxide, potassium cyanide. Insoluble in water, ethanol, hydrochloric acid ,dilute acids, sulfuric acid, alkali cyanides, aqueous ammonia and alkaline solutions. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,2965 |
| Solubility Product Constant (Ksp) | pKsp: 35.2 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Stability: | Stable, but may be moisture senstive. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 1317-40-4(CAS DataBase Reference) |
| EPA Substance Registry System | Copper sulfide (CuS) (1317-40-4) |
Safety information for COPPER(II) SULFIDE
Computed Descriptors for COPPER(II) SULFIDE
COPPER(II) SULFIDE manufacturer
Arihant Purechem Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran

You may like
-
 Copper(II) sulfide, Ultra dry CAS 1317-40-4View Details
Copper(II) sulfide, Ultra dry CAS 1317-40-4View Details
1317-40-4 -
 Copper(II) sulfide, Ultra dry CAS 1317-40-4View Details
Copper(II) sulfide, Ultra dry CAS 1317-40-4View Details
1317-40-4 -
 Copper(II) sulfide CAS 1317-40-4View Details
Copper(II) sulfide CAS 1317-40-4View Details
1317-40-4 -
 Copper(II) sulfide CAS 1317-40-4View Details
Copper(II) sulfide CAS 1317-40-4View Details
1317-40-4 -
 Copper(II) sulfide CAS 1317-40-4View Details
Copper(II) sulfide CAS 1317-40-4View Details
1317-40-4 -
 Copper(II)Sulfide 98% CAS 1317-40-4View Details
Copper(II)Sulfide 98% CAS 1317-40-4View Details
1317-40-4 -
 Copper(II) sulfide CAS 1317-40-4View Details
Copper(II) sulfide CAS 1317-40-4View Details
1317-40-4 -
 Copper(II) sulfide CAS 1317-40-4View Details
Copper(II) sulfide CAS 1317-40-4View Details
1317-40-4