7758-99-8
Product Name:
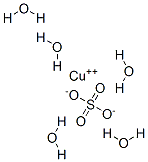
Copper(II) sulfate pentahydrate
Formula:
CuH10O9S
Synonyms:
Copper(II) sulfate pentahydrate;Cupric sulfate pentahydrate;Cupric sulfate standard;Sulfuric acid, copper(II) salt (1:1) pentahydrate;Blue Vitriol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Copper sulfate pentahydrate appears as blue crystalline granules or powder. Melting point 110 °C (with decomposition). Non-combustible. Nauseating metallic taste. Odorless. White when dehydrated. (NTP, 1992) |
|---|---|
| Color/Form | Large, blue or ultramarine, triclinic crystals or blue granules or light-blue powder |
| Odor | Odorless |
| Taste | Nauseous, metallic taste |
| Boiling Point | 1207 °F at 760 mmHg (decomposes) (NTP, 1992) |
| Melting Point | 297 °F (dehydrates) (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 70 °F (NTP, 1992) |
| Density | 2.284 (NTP, 1992) - Denser than water; will sink |
| Stability/Shelf Life | INDEFINITE WHEN KEPT DRY; STABLE TO HEAT, COLD, & LIGHT. |
| Decomposition | DECOMP ABOVE 150 °C (BOILING POINT) WITH -5H2O /SPR: TO ANHYDROUS SALT/ |
| Corrosivity | Very corrosive to plain steel. |
| pH | pH of 0.2 molar aq soln: 4.0 |
| Refractive Index | INDICES OF REFRACTION: 1.514, 1.537, 1.543 |
| Other Experimental Properties | Loses 2H2O @ 30 °C, 2 more H2O @ 110 °C; becomes anhydrous by 250 °C; efflorescent in air. |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 249.69 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 0 |
| Exact Mass | 248.934150 g/mol |
| Monoisotopic Mass | 248.934150 g/mol |
| Topological Polar Surface Area | 93.6 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 7 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Copper sulfate pentahydrate appears as blue crystalline granules or powder. Melting point 110 °C (with decomposition). Non-combustible. Nauseating metallic taste. Odorless. White when dehydrated. (NTP, 1992). It has been used: as an additive in trace element solution preparation in solid glucose minimal media. as a component of adamsII solution in Pneumococcal media. in the preparation of alginate gel for drug encapsulation.