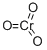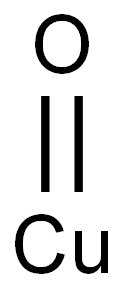Copper chromite
- CAS NO.:12053-18-8
- Empirical Formula: CrCuH2O
- Molecular Weight: 133.56
- MDL number: MFCD00044868
- EINECS: 235-000-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-22 18:19:53
What is Copper chromite?
Description
Copper chromite is a black powder and inorganic pigment thatadopts a spinel crystal structure. It can be made by the high-temperaturecalcination of copper(II) oxide and chromium(III) oxide or the thermaldecomposition of copper chromate, which evolves oxygen at higher temperaturesand decomposes to copper chromite.
The copper chromite (CuCr2O4) is one of the most efficient materials, has wide commercial application as catalysts being used in the unit processes of organic synthesis such as hydrogenation, dehydrogenation, hydrogenolysis, oxidation, alkylation, cyclization, etc. It can be used in the pollution abatement as the catalyst to remove aqueous organic wastes, volatile organic compound (VOC) and vehicular primary emissions such as CO, unburned hydrocarbon, NOx and soot.
Chemical properties
black powder
The Uses of Copper chromite
Copper chromite is primarily used as a catalyst forhydrogenation reactions because of its ability to hydrogenate functional groupsin aliphatic and aromatic compounds selectively. Industrially, copper chromiteis used to reduce furfural to furfuryl alcohol and butyraldehyde to 1-butanol,partially reduce conjugated dienes to monoenes, and selectively reduce carbonylgroups in vegetable oils. This CuCr catalyst is studied for a variety ofcatalytic applications including converting the hydrogenolysis of cellulose.It is also used as a catalyst for combustion to help control burn rate and as alight-absorbing pigment.
Preparation
Copper chromite is produced by thermal decomposition of one of three substances. The traditional method is by the ignition of copper chromate:
2CuCrO4 → 2CuCrO3 + O2
Copper barium ammonium chromate is the most commonly used substance for production of copper chromite. The resulting copper chromite mixture produced by this method can only be used in procedures that contain materials inert to barium, as barium is a product of the decomposition of copper barium ammonium chromate, and is thus present in the resulting mixture. The by-product copper oxide is removed using an acetic acid extraction, consisting of washing with the acid, decantation and then heat drying of the remaining solid to yield isolated copper chromite. Copper chromite is produced by the exposure of copper barium ammonium chromate to temperatures of 350-450 °C, generally by a muffle furnace:
Ba2Cu2(NH4)2(CrO4)5 → CrCuO3 + CuO + 2Ba + 4H2O + 4Cr + N2 + 6O2
Properties of Copper chromite
| form | powder |
| InChI | InChI=1S/Cr.Cu.H2O/h;;1H2 |
Safety information for Copper chromite
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Copper chromite
| InChIKey | ZKEGZFIDFXEQLM-UHFFFAOYSA-N |
| SMILES | [Cr].[Cu].O |
Copper chromite manufacturer
Anand Agencies
ASM Organics
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 12053-18-8 COPPER CHROMITE CATALYST 99%View Details
12053-18-8 COPPER CHROMITE CATALYST 99%View Details
12053-18-8 -
 12053-18-8 99%View Details
12053-18-8 99%View Details
12053-18-8 -
 Copper chromite CAS 12053-18-8View Details
Copper chromite CAS 12053-18-8View Details
12053-18-8 -
 Copper chromite CAS 12053-18-8View Details
Copper chromite CAS 12053-18-8View Details
12053-18-8 -
 Copper chromite CAS 12053-18-8View Details
Copper chromite CAS 12053-18-8View Details
12053-18-8 -
 Copper chromite, 40% 99%View Details
Copper chromite, 40% 99%View Details
12053-18-8 -
 Copper chromite CAS 12053-18-8View Details
Copper chromite CAS 12053-18-8View Details
12053-18-8 -
 Copper chromite CAS 12053-18-8View Details
Copper chromite CAS 12053-18-8View Details
12053-18-8