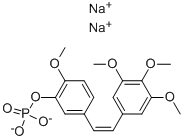
Combretastatin A4 phosphate disodium salt
Synonym(s):2-Methoxy-5-[(1Z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenyl disodium phosphate;CA4P;Combretastatin A4 phosphate disodium
- CAS NO.:168555-66-6
- Empirical Formula: C18H19O8P.2Na
- Molecular Weight: 440.29
- MDL number: MFCD00943911
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Combretastatin A4 phosphate disodium salt?
Description
Combretastatin A4 phosphate disodium salt, also known as fosbretabulin disodium or CA4P, is a microtubule destabilizing drug, a water-soluble prodrug of combretasain a4(ca4) and a tumor vascular-targeting agent.microtubule is the cylindrical and filamentous structure that required for cell shape, migration, cilia and flagella mobility etc. tubulin is the major component of microtubulesin proliferative endothelial cells, ca4p (dose less than 1/10 of the max. tolerated dose) leaded to tumor vascular shutdown, and short drug exposure of ca4p caused significant long-term anti-proliferative and cytotoxic effects. [1] in ca40 treated endothelia cell, myosin light chain was phosphorylated and lead to actinomyosin contractility, assembly of actin stress fibers and focal adhesions formation. [2] in huvec cells, ca4p blocked growth factor–induced endothelial cell proliferation and migration and impaired capillary tube formation. [3]in c57bl/6 mice, ca4p treatment in combination with mab against ve-cardherin facilitated b16 melanoma tumor necrosis and inhibited tumor neoangionesis. [3] in human breast cancer models in vivo, 6 hours after ca4p administration showed a 93% decreased functional vascular volume and lasted over the next 12 hours. [1]
Chemical properties
White Solid
The Uses of Combretastatin A4 phosphate disodium salt
Phosphorylated Combretastatin, a small organic molecule found in the bark of the African bush willow tree Combretum caffrum. Combretastatin-A4 Prodrug Induces Mitotic Catastrophe in Chronic Lymphocytic Leukemia Cell Line Independent of Caspase Activation and Poly(ADP-ribose) Polymerase Cleavage. A potent cytotoxic agent which strongly inhibits the polymerization of tubulin by binding to the colchicine site.
What are the applications of Application
Combretastatin A4 Phosphate Disodium Salt is A microtubule depolymerizing molecule
Preparation
Combretastatin A-4 phosphate can be synthesized by phosphorylation of combretastatin A-4 (I) following several related strategies. Direct phosphorylation of (I) employing either bis(2,2,2-trichloroethyl) phosphorochloridate in pyridine (1,2) or dibenzyl phosphite in the presence of CCl4 and DMAP (3-5) furnishes the corresponding bis-trichloroethyl (IIa) and dibenzyl (IIb) phosphate esters, which are subsequently deprotected to combretastatin A-4 phosphate (III) by either reductive trichloroethyl group cleavage with zinc dust and acetic acid (1,2) or by debenzylation with chlorotrimethylsilane/sodium iodide (3-5). Alternatively, phosphitylation of (I) using di-tert-butyl N,N- diethylphosphoramidite (3,4,6) or bis(trimethylsilylethyl) N,N-diisopropylphosphoramidite (3-5) in the presence of tetrazole yields the phosphite esters (IVa) and (IVb), respectively, which are further oxidized to the corresponding phosphates (Va) and (Vb) using m-chloroperbenzoic acid in CH2Cl2/THF (3-6). Combretastatin A-4 phosphate (III) is then obtained by acidic cleavage of the tert-butyl ester (Va) with trifluoroacetic acid (3,4,6) or trifluoromethanesulfonic acid (6), or by tetrabutylammonium fluoride-promoted cleavage of the trimethylsilylethyl ester (Vb) (3-5).
Biochem/physiol Actions
Fosbretabulin disodium (Combretastatin A4 disodium phosphate, CA4P) is a potent vascular-disrupting agent (VDA). Fosbretabulin disodium is a prodrug that is converted to combretastatin A inside the endothelial cells that line blood vessels, where it binds to tubulin dimers and prevents microtubule polymerization, resulting in mitotic arrest and apoptosis in endothelial cells. Fosbretabulin disodium is a potent anti-cancer agent that exhibits antivascular effects on tumor vasculature, inducing a rapid reduction in tumor blood flow and a concomitant increase of cellular necrosis. Some of its activity is believed to involve interference with vascular endothelial-cadherin signaling in addition to its microtubule-disrupting activity.
Mode of action
Combretastatin A4 disodium phosphate works by quickly interrupting the blood supply to tumors, resulting in tumor death due to lack of oxygen and nutrients. Its mechanism of action in treating tumors is distinct from general cytotoxic chemotherapy drugs and drugs that inhibit the formation of new blood vessels. Unlike these drugs, Compradine Disodium Phosphate targets and breaks down the immature endothelial cells of newly formed blood vessels during tumor growth, while having no effect on the mature endothelial cells of normal tissue blood vessels.
References
[1] dark gg, hill sa, prise ve, tozer gm, pettit gr, chaplin dj. combretastatin a-4, an agent that displays potent and selective toxicity toward tumor vasculature. cancer res. 1997 may 15;57(10):1829-34.
[2] KANTHOU C, TOZER G M. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells.[J]. Blood, 2002, 99 6: 2060-2069. DOI:10.1182/BLOOD.V99.6.2060.
[3] VINCENT L, KERMANI P, YOUNG L M, et al. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling.[J]. The Journal of clinical investigation, 2005, 115 11: 2992-3006. DOI:10.1172/JCI24586.
[4] PRABHU S, HARRIS F, LEA R W, et al. Small-molecule clinical trial candidates for the treatment of glioma.[J]. Drug discovery today, 2014, 19 9: 1298-1308. DOI:10.1016/j.drudis.2014.02.007.
[5] TOCHINAI R, NAGATA Y, ANDO M, et al. Combretastatin A4 disodium phosphate-induced myocardial injury[J]. Journal of Toxicologic Pathology, 2016, 29: 163-171. DOI:10.1293/tox.2016-0012.
[6] PATTERSON D M, RUSTIN G J S, SERRADELL N, et al. Combretastatin A-4 phosphate[J]. Drugs of The Future, 2007, 32: 1025. DOI:10.1358/DOF.2007.032.12.1158960.
Properties of Combretastatin A4 phosphate disodium salt
| Melting point: | 238-242 ºC |
| storage temp. | -20°C |
| solubility | H2O: soluble5mg/mL, clear |
| form | powder |
| color | white to beige |
| CAS DataBase Reference | 168555-66-6 |
Safety information for Combretastatin A4 phosphate disodium salt
Computed Descriptors for Combretastatin A4 phosphate disodium salt
| InChIKey | VXNQMUVMEIGUJW-XNOMRPDFSA-L |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran

You may like
-
 Combretastatin A4 Phosphate Disodium Salt CAS 168555-66-6View Details
Combretastatin A4 Phosphate Disodium Salt CAS 168555-66-6View Details
168555-66-6 -
 Combretastatin a4 disodium phosphate 95% CAS 168555-66-6View Details
Combretastatin a4 disodium phosphate 95% CAS 168555-66-6View Details
168555-66-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8