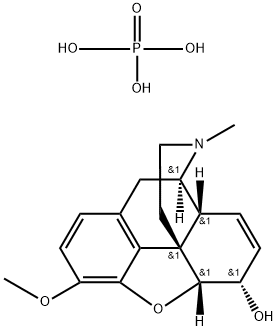
CODEINE PHOSPHATE
- CAS NO.:41444-62-6
- Empirical Formula: C18H24NO7P
- Molecular Weight: 397.36
- MDL number: MFCD00153030
- EINECS: 639-473-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is CODEINE PHOSPHATE?
Chemical properties
White or almost white, crystalline powder or small, colourless crystals.
Originator
Actacode Linctus,Sigma
The Uses of CODEINE PHOSPHATE
Antitussive; analgesic (narcotic).
brand name
Ambenyl Cough Syrup (Parke-Davis); Colrex Compound (Solvay Pharmaceuticals).
Therapeutic Function
Narcotic analgesic, Antitussive
Clinical Use
Codeine is used extensively to treat moderate to mild pain. Codeine is a weak μ agonist, but approximately 10% of an oral dose (30–60 mg) is metabolized to morphine, which contributes significantly to its analgesic effect. The plasma half-life of codeine after oral dose is 3.5 hours. The dose of codeine needed to produce analgesia after parenteral dose causes releases of histamine sufficient to produce hypotension, pruritus, and other allergic responses. Thus, administration of codeine by parenteral route is not recommended.
Veterinary Drugs and Treatments
In small animal medicine, codeine is used principally as an oral analgesic when salicylates are not effective and parenteral opiates are not warranted. It may also be useful as an antitussive or an antidiarrheal.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism increased by rifampicin.
Antidepressants: possible CNS excitation or
depression with MAOIs - avoid concomitant use,
and for 2 weeks after stopping MAOI; possible
CNS excitation or depression with moclobemide;
increased sedative effects with tricyclics.
Antihistamines: increased sedative effects with
sedating antihistamines.
Antipsychotics: enhanced hypotensive and sedative
effects.
Dopaminergics: avoid with selegiline.
Nalmefene: avoid concomitant use.
Sodium oxybate: enhanced effect of sodium oxybate
- avoid.
Metabolism
Codeine is metabolised by O- and N-demethylation in
the liver to morphine, norcodeine, and other metabolites
including normorphine and hydrocodone. Metabolism to
morphine is mediated by the cytochrome P450 isoenzyme
CYP2D6, which shows genetic polymorphism.
Codeine and its metabolites are excreted almost entirely
by the kidney, mainly as conjugates with glucuronic acid.
Properties of CODEINE PHOSPHATE
| solubility | Freely soluble in water, slightly soluble or very slightly soluble in ethanol (96 per cent). |
| form | A crystalline solid |
| CAS DataBase Reference | 41444-62-6 |
Safety information for CODEINE PHOSPHATE
Computed Descriptors for CODEINE PHOSPHATE
CODEINE PHOSPHATE manufacturer
New Products
3-pyridine carboxaldehyde 7-bromoquinoxalin-2(1H)-one 2-Bromo-6-flurobenzaldehyde 8-Bromoisoquinoline 3-Pyridineacetonitrile, α-amino-, hydrochloride (1:1) Ethyl 3-Phenoxypropionate 4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE TosMIC 2-Iodo-5-methoxybenzoic acid Xanomeline Suzetrigine Remibrutinib Dimethyl 4-aminothiophene-2,3-dicarboxylate hydrochloride 1H-Indole-1-carboxylic acid, 5-hydroxy-7-methyl-, 1,1-dimethylethyl ester N N N'Trimethyl ethylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N' DimethylEthylenediamine Ethyl Methanesulfonate N Ethylmethylamine Radiator Flux Zinc Chloride Solution (All Grades) Calcium Alphaketoglutarate* Fmoc-L-Tyr(tBu)-OHRelated products of tetrahydrofuran



![MORPHINE SULPHATE IMP. A (EP) AS PHOSPHATE HEMIHYDRATE: CODEINE PHOSPHATE HEMIHYDRATE[CONTROLLED SUBSTANCE]](https://img.chemicalbook.in/)
You may like
-
 654655-69-3 3-Benzyl-6-bromo-2- methoxy quinoline 99%View Details
654655-69-3 3-Benzyl-6-bromo-2- methoxy quinoline 99%View Details
654655-69-3 -
 1181267-33-3 99%View Details
1181267-33-3 99%View Details
1181267-33-3 -
 2,4-Difluoro-alpha-(1H- 1,2,4,Triazolyl)acetophenone 99%View Details
2,4-Difluoro-alpha-(1H- 1,2,4,Triazolyl)acetophenone 99%View Details
86404-63-9 -
 1076-22-8 3-Methyl xanthine 99%View Details
1076-22-8 3-Methyl xanthine 99%View Details
1076-22-8 -
 161596-47-0 99%View Details
161596-47-0 99%View Details
161596-47-0 -
 3-Amino-4-pyrazole carboxamide hemisulfate 27511-79-1 99%View Details
3-Amino-4-pyrazole carboxamide hemisulfate 27511-79-1 99%View Details
27511-79-1 -
 3-Dimethl amino-1- (naphthalen-1-yl)propan-1- one hydro chloride 99%View Details
3-Dimethl amino-1- (naphthalen-1-yl)propan-1- one hydro chloride 99%View Details
5409-58-5 -
 109113-72-6 2-(chloro methyl)-4-methyl quinazoline 99%View Details
109113-72-6 2-(chloro methyl)-4-methyl quinazoline 99%View Details
109113-72-6
