COBALT(II) IODIDE
Synonym(s):Cobalt diiodide;Cobalt iodide;Cobaltous iodide
- CAS NO.:15238-00-3
- Empirical Formula: CoI2
- Molecular Weight: 312.74
- MDL number: MFCD00016030
- EINECS: 239-283-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-29 14:23:27
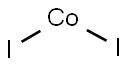
What is COBALT(II) IODIDE?
Description
Cobalt iodide appears as black crystals with a slight ‘iodine’-like odour. When heated to decomposition, it may emit toxic fumes of iodine and oxides of nitrogen. Anhydrous cobalt (II) iodide is sometimes used to test for the presence of water in various solvents. Cobalt (II) iodide is used as a catalyst, for example, in carbonylations. It catalyses the reaction of diketene with Grignard reagents, useful for the synthesis of terpenoids.
Chemical properties
Brownish red crystalline powder
Physical properties
Exists in two isomorphous forms, α- and ?-forms; both modifications highly hygroscopic. The α-form is black hexagonal crystal; density 5.58 g/cm3; turns dark green in air; melts at 560°C; disolves in water giving pink coloration. The α-forms sublimes in vacuo, partly forming an isomorous yellow modification-the anhydrous β-form.
The β-modification is a yellow powder; density 5.45 g/cm3; converts to the α-form when heated to 400°C; absorbs moisture from air, the yellow powder becoming green droplets; dissolves readily in water forming a colorless solution which turns pink on heating.
The hexahydrate is red hexagonal crystals; density 2.90 g/cm3; loses water at 130°C giving anhydrous iodide; soluble in water, ethanol, acetone, chloroform and ether, forming colored solutions, (while the aqueous solution is red below 20°C and green above this temperature; the salt forms blue solution in ethanol, chloroform and ether).
The Uses of COBALT(II) IODIDE
Cobalt(II) iodide is used as a moisture and humidity indicator. It is also used as a catalyst.
The Uses of COBALT(II) IODIDE
Hygrometers; determination of water in organic solvents; catalyst.
What are the applications of Application
Cobalt(II) iodide is a moisture and humidity indicator
Preparation
Cobalt(II) iodide is prepared by heating cobalt powder in a stream of hydrogen iodide at 400 to 450°C:
Co + 2HI → CoI2 + H2
The product obtained is the black crystalline α-form.
Cobalt(II) iodide also may be made by heating cobalt powder with iodine vapor.
Structure and conformation
Cobalt(II) iodide crystallizes in two polymorphs, the α- and β-forms. The α-polymorph comprises black hexagonal crystals, which turn dark green when exposed to air. Under vacuum at 500℃, samples of α-CoI2 sublime, forming the β-polymorph as yellow crystals. β-CoI2 also easily absorbs moisture from the air, converting into green hydrate. Below 400℃, β-CoI2 reverts back to the α-form. The anhydrous salt has the cadmium halide structure. The hexaaquo salt comprises separated [Co(H2O)6] 21 and iodide ions.
Properties of COBALT(II) IODIDE
| Melting point: | >515 °C(lit.) |
| Boiling point: | 570°C |
| Density | 5.45 g/mL at 25 °C(lit.) |
| Flash point: | 570°C vac. |
| solubility | acetone: slightly soluble(lit.) |
| form | powder |
| Specific Gravity | 5.68 |
| color | White |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,2443 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 15238-00-3(CAS DataBase Reference) |
| EPA Substance Registry System | Cobalt iodide (CoI2) (15238-00-3) |
Safety information for COBALT(II) IODIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for COBALT(II) IODIDE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Cobalt(II) iodide, Ultra dry CAS 15238-00-3View Details
Cobalt(II) iodide, Ultra dry CAS 15238-00-3View Details
15238-00-3 -
 Cobalt(II) iodide, Ultra dry CAS 15238-00-3View Details
Cobalt(II) iodide, Ultra dry CAS 15238-00-3View Details
15238-00-3 -
 Cobalt(II) iodide, Anhydrous CAS 15238-00-3View Details
Cobalt(II) iodide, Anhydrous CAS 15238-00-3View Details
15238-00-3 -
 Cobalt(II) iodide, Anhydrous CAS 15238-00-3View Details
Cobalt(II) iodide, Anhydrous CAS 15238-00-3View Details
15238-00-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4