Clozapine
Synonym(s):8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]-diazepine;Clozapine
- CAS NO.:5786-21-0
- Empirical Formula: C18H19ClN4
- Molecular Weight: 326.82
- MDL number: MFCD00153785
- EINECS: 227-313-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
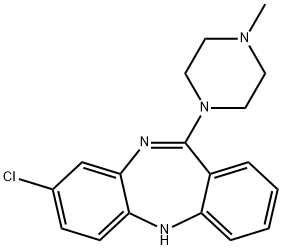
What is Clozapine?
Absorption
In humans, clozapine tablets (25 mg and 100 mg) are equally bioavailable relative to a CLOZARIL solution. Following oral administration of clozapine 100 mg twice daily, the average steady-state peak plasma concentration was 319 ng/mL (range: 102 to 771 ng/mL), occurring at the average of 2.5 hours (range: 1 to 6 hours) after dosing. The average minimum concentration at steady state was 122 ng/mL (range: 41 to 343 ng/mL), after 100 mg twice daily dosing.
Toxicity
There are no adequate or well-controlled studies of clozapine in pregnant women. Reproduction studies have been performed in rats and rabbits at doses up to 0.4 and 0.9 times, respectively, the maximum recommended human dose (MRHD) of 900 mg/day on a mg/m2 body surface area basis. The studies revealed no evidence of impaired fertility or harm to the fetus due to clozapine. Because animal reproduction studies are not always predictive of human response, CLOZARIL should be used during pregnancy only if clearly needed.
Consider the risk of exacerbation of psychosis when discontinuing or changing treatment with antipsychotic medications
during pregnancy and postpartum. Consider early screening for gestational diabetes for patients treated with antipsychotic
medications [see Warnings and Precautions (5.11)]. Neonates exposed to antipsychotic drugs during the third trimester of
pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. Monitor neonates for symptoms
of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding difficulties. The severity of
complications can vary from self-limited symptoms to some neonates requiring intensive care unit support and prolonged
hospitalization.
The most commonly reported signs and symptoms associated with clozapine overdose are: sedation, delirium, coma,
tachycardia, hypotension, respiratory depression or failure; and hypersalivation. There are reports of aspiration
pneumonia, cardiac arrhythmias, and seizure. Fatal overdoses have been reported with clozapine, generally at doses above
2500 mg. There have also been reports of patients recovering from overdoses well in excess of 4 g. There is no available specific antidote to an overdose of CLOZARIL. Establish and maintain an airway; ensure adequate oxygenation and ventilation. Monitor cardiac status and vital signs. Use general symptomatic and supportive measures. Consider the possibility of multiple-drug involvement.
No carcinogenic potential was demonstrated in long-term studies in mice and rats at doses up to 0.3 times and 0.4 times,
respectively, the maximum recommended human dose (MRHD) of 900 mg/day on an mg/m2 body surface area basis. Clozapine was not genotoxic when tested in the following gene mutation and chromosomal aberration tests: the bacterial Ames test, the in vitro mammalian V79 in Chinese hamster cells, the in vitro unscheduled DNA synthesis in rat hepatocytes or the in vivo micronucleus assay in mice. Clozapine had no effect on any parameters of fertility, pregnancy, fetal weight, or postnatal development when
administered orally to male rats 70 days before mating and to female rats for 14 days before mating at doses up to 0.4 times the MRHD of 900 mg/day on an mg/m2 body surface area basis.
Description
Clozapine (5786-21-0) is a dopamine D4?and D2?receptor antagonist. High affinity for the cloned rat dopamine D4?receptor (Ki?< 20 nM).1?Atypical neuroleptic agent.2?Antagonist at 5HT2A, 5HT2C, 5HT3, 5HT6 and 5HT7 receptors.3,4
Chemical properties
Yellow Crystalline Solid
Originator
Leponex,Wander,W. Germany,1974
The Uses of Clozapine
Clozapine is a neuroleptic, which expresses antipsychotic and sedative action. It does not cause general depression and extrapyramidal disorders. It is used for severe and chronic forms of schizophrenia, maniacal conditions, manic-depressive psychosis, psychomotor excitement, and various other psychotic conditions.
The Uses of Clozapine
An antipsychotic
The Uses of Clozapine
depigmentor
What are the applications of Application
Clozapine is a selective inhibitor for D4-dopamine and several serotonin receptors
Background
Clozapine is a tricyclic dibenzodiazepine, classified as an atypical antipsychotic agent. Clozapine displays affinity to various neuroreceptors with a particularly low affinity to the dopamine receptors, thus breaking the mold of first-generation antipsychotics and deeming it "atypical".. This low affinity to dopamine receptors results in fewer extrapyramidal side effects, especially tardive dyskinesia. However, its promiscuity toward the muscarinic and adrenergic receptors can result in other side effects, notably gastrointestinal hypomotility and orthostatic hypotension. . Despite its effectiveness in treating both positive and negative symptoms of schizophrenia, clozapine was briefly removed from the market in various jurisdictions in 1970 due to severe agranulocytosis. However, continued evidence of its effectiveness led to clozapine's eventual reintroduction, although with a reluctance to prescribe it.
Clozapine was approved by the FDA in 1989 for treatment-resistant schizophrenia under the brand CLOZARIL. Due to its severe adverse effects profile, clozapine is only available through a restricted program under a Risk Evaluation Mitigation Strategy (REMS) called the Clozapine REMS Program.
Indications
Clozapine is indicated for the treatment of severely ill patients with schizophrenia who fail to respond adequately to standard antipsychotic treatment. Because of the risks of severe neutropenia and of seizure associated with its use, Clozapine should be used only in patients who have failed to respond adequately to standard antipsychotic treatment.
Clozapine is also indicated for reducing the risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for re-experiencing suicidal behavior, based on history and recent clinical state. Suicidal behavior refers to actions by a patient that put him/herself at risk for death.
Definition
ChEBI: A benzodiazepine that is 5H-dibenzo[b,e][1,4]diazepine substituted by a chloro group at position 8 and a 4-methylpiperazin-1-yl group at position 11. It is a second generation antipsychotic used in the treatment of psychiatr c disorders like schizophrenia.
Manufacturing Process
7.4 g of 2-amino-4-chlorodiphenylamine-2'-carboxylic acid (4"-methyl) piperazide and 35 ml of phosphoroxychloride are heated for 3 hours under reflux in the presence of 1.4 ml of N,N-dimethylaniline. Upon concentration of the reaction mixture in vacuo as far as possible, the residue is distributed between benzene and ammonia/ice water. The benzene solution is extracted with dilute acetic acid. The acid extract is clarified with charcoal and treated with concentrated ammonia water to precipitate the alkaline substance, which is dissolved in ether. The ethereal solution is washed with water and dried over sodium sulfate. The residue obtained yields, after recrystallization from ether/petroleum ether 2.9 g (41% of the theoretical yield) of 8-chloro-11-(4- methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine in the form of yellow grains of melting point 182° to 184°C (from acetone/petroleum ether).
brand name
Clozaril (Novartis); Fazaclo (Avanir);Leponox.
Therapeutic Function
Tranquilizer
World Health Organization (WHO)
Clozapine, a tricyclic neuroleptic, was introduced in 1972 for the treatment of psychosis. In 1975 its use was associated with cases of agranulocytosis, particularly in Finland. These cases, which included several fatalities, resulted in the withdrawal of the drug in some countries. However, clozapine remains available in at least 30 countries, in some cases only on special request, for the treatment of severe psychotic disorders unresponsive to other neuroleptics provided that close monitoring of the blood count is feasible. In 1989, it was introduced in the United States for the treatment of severe schizophrenia. Lately, the use of clozapine in the United Kingdom has been associated with convulsions. (Reference: (WHODIB) WHO Drug Information Bulletin, 2: 10, , 1977)
General Description
Clozapine, 8-chloro-11-(4-methyl-1-piperazinyl)5H-dibenzo[b,e] [1,4] diazepine (Clozaril), is a yellowcrystalline powder that is only slightly soluble in water. Withthe introduction of clozapine, a different pharmacological antipsychotics.106 Unlike typical antipsychotics, clozapine is largelydevoid of EPS. The lack of EPS with this compound waspostulated to be caused by its preferential binding tomesolimbic rather than striatal DA receptors.Furthermore,clozapine was shown to exhibit potent affinity for 5-HT2Areceptors.
DMCZ shows partial agonism at D2 and D3 receptors andexhibits a distinctly different pharmacological profile comparedwith clozapine and other atypical antipsychotics.Unlike clozapine, which is a potent M1 muscarinic antagonist,DMCZ is a potent M1 agonist. Agonism at muscarinicreceptors has been proposed to be useful for impaired cognitionin schizophrenia. Burnstein et al.found thatDMCZ acted as a partial agonist at DA D2 and D3 receptors.These investigators suggested that the low incidence of EPSassociated with clozapine may be caused by the partial agonismof DMCZ at D2 and D3 receptors. Thus, DMCZ maybe of interest as an atypical antipsychotic with an improvedside effect profile compared with clozapine.
General Description
The dibenzodiazepine derivative is clozapine(Clozaril). It is not a potent antipsychotic on a milligrambasis (note the orientation of the N-methyl piperazino grouprelative to the chlorine atom). In addition to their moderatepotencies at DA receptors (mainly D4), clozapine interactwith varying affinities at several other classes of receptors(α1 and α2 adrenergic, 5-HT1A, 5-HT2A, 5-HT2C, muscariniccholinergic, histamine H1, and others). It is effective againstboth positive and negative symptoms of schizophrenia andhas a low tendency to produce EPS. Clozapine has proved effectiveeven in chronically ill patients who respond poorly tostandard neuroleptics. However, there are legal restrictionson its use because of a relatively high frequency of agranulocytosis.As a rule, two other antipsychotics are tried beforerecourse to therapy with clozapine. Clozapine is metabolized preferentially by CYP3A4 into demethylated, hydroxylated,and N-oxide derivatives that are excreted in urine and feces.Elimination half-life averages about 12 hours. Other clozapine-like atypical antipsychotics may lack a 2-Cl substituenton the aromatic ring (e.g., olanzapine and quetiapine).
Biological Activity
Atypical antipsychotic drug, with a much lower tendency to cause extrapyramidal side effects than conventional neuroleptics. Displays a broad range of pharmacological actions; the antipsychotic effects are thought to be mediated principally by 5-HT 2A/2C and dopamine receptor blockade (K i values are 21, 170, 170, 230 and 330 nM for D 4 , D 3 , D 1 , D 2 and D 5 receptors respectively).
Biochem/physiol Actions
Atypical antipsychotic compound. Selective antagonist for D4-dopamine receptor. Antagonist at 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, and 5-HT7 serotonin receptors.
Pharmacokinetics
Clozapine is a psychotropic agent belonging to the chemical class of benzisoxazole derivatives that is universally regarded as the treatment of choice for treatment-resistant schizophrenia. Although it is thought to mediate its pharmacological effect through antagonism of the dopamine type 2 (D2) and the serotonin type 2A (5-HT2A) receptors, research have shown that clozapine can act on various types of receptors.
Patients should be counseled regarding the risk of hypersensitivity reactions such as agranulocytosis and myocarditis with clozapine use. Clozapine-induced agranulocytosis, which is a reduction in the absolute neutrophil count or white blood cell count, places the patient at an increased risk for infection. Agranulocytosis is most likely to occur in the first 3-6 months of therapy, but it can still occur after years of treatment. The mechanism is thought to be a dose-independent and immune-mediated reaction against neutrophils. Patients are strictly monitored by lab testing (complete blood count with differential) to ensure agranulocytosis is detected and treated if it occurs. Testing is initially completed at one-week intervals but is expanded to two-week intervals at six months, and then four-week intervals at twelve months if lab results have been within an appropriate range. Monitoring parameters may change if there is any break in therapy. In Canada, the patient's lab values are reported to the manufacturer for hematological monitoring, and in the USA, the patient's lab values are reported to the REMS (Risk Evaluation and Mitigation Strategy) program. These programs function to notify the care provider of any significant drop in WBC/neutrophil count, or if there is a drop below a threshold level. Patients who enter the "Red" zone (WBC<2x109/L or ANC<1.5x109/L) should normally not be re-challenged.
Clozapine-induced myocarditis is a hypersensitivity reaction that usually occurs in the third week of clozapine therapy and about 2% of clozapine patients. Monitor the patient's troponin, CRP, and ECG at baseline, and 28 days into treatment. Follow guidelines for appropriate next steps according to the patient's lab results. If myocarditis occurs, the patient should not be re-challenged with clozapine.
Clinical Use
Although clozapine demonstrates a favorable pharmacologicalprofile compared with typical antipsychotics, its useis restricted by a relatively high incidence of agranulocytosis.The exact mechanism for the cause of agranulocytosishas not been confirmed, but a highly reactive nitrenium ionthat is formed by the action of hepatic enzymes appears tobe involved.The mean elimination half-life of clozapine following a single 75-mg dose is 8 hours. Because of severaladverse effects, clozapine is only used in refractory casesof schizophrenia. Individuals with a history of seizures orpredisposed to seizures should be cautioned when takingclozapine. Similar to other atypical antipsychotic agents,clozapine causes an increased risk of mortality in elderly individualswith dementia-related psychoses.
Side Effects
A serious drawback to the use of clozapine, however, is the potentially fatal agranulocytosis that is reported to occur in 1 to 2% of unmonitored patients, necessitating weekly white blood cell counts for at least the first 6 months of pharmacotherapy.
Synthesis
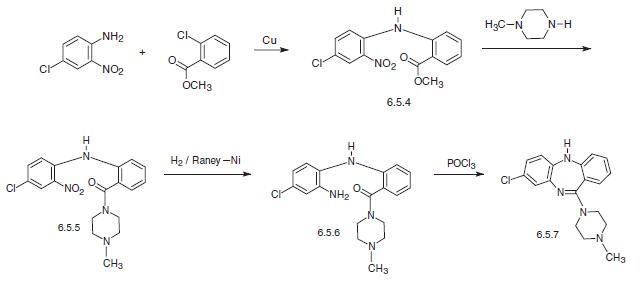
Clozapine, 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e] [1,4]diazepine (6.5.7) is synthesized by two methods. According to the first, 4-chloro-2-nitroaniline in the presence of copper filings is acylated by the o-chlorobenzoic acid methyl ester, forming the corresponding diphenylamine (6.5.4). By reacting this with N-methyl piperazine, the ester group in the resulting polyfunctional diphenylamine is transformed into the amide (6.5.5). The nitro group in the resulting 4-chloro-2- nitro-2′-carb-(N′-methyl piperazino)amide (6.5.5) is further reduced into an amine group by hydrogen in the presence of Raney nickel. Reacting the resulting product (6.5.6) with phosphorous oxychloride yields in heterocyclization into the desired dibenzodiazepine, clozapine (6.5.7).
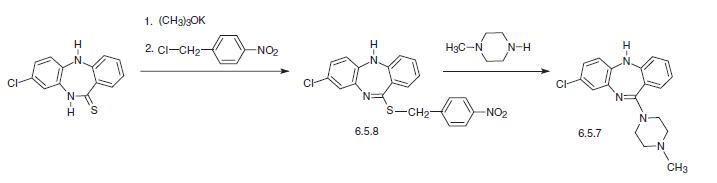
The other way of synthesis of clozapine is from 8-chloro-10,11-dihydro-5H-dibenzo[b,e]1, 4-diazepin-11-thione, which is alkylated at the sulfur atom of the dibenzodiazepine ring by 4-nitrobenzylchloride in the presence of potassium tert-butoxide, giving N-methyl derivative (6.5.8). Reaction of this with N-methylpiperazine gives the desired clozapine (6.5.7).
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong
the QT interval; increased risk of arrhythmias with
flecainide.
Antibacterials: concentration possibly increased
by erythromycin (possible increased risk
of convulsions); concentration increased by
ciprofloxacin; concentration possibly reduced
by rifampicin; avoid with chloramphenicol and
sulphonamides (increased risk agranulocytosis).
Antidepressants: concentration possibly increased
by citalopram, fluoxetine, fluvoxamine, paroxetine,
sertraline and venlafaxine (increased risk of toxicity);
possibly increased CNS effects of MAOIs; possibly
increased antimuscarinic effects with tricyclics;
increased concentration of tricyclics.
Antiepileptics: antagonises anticonvulsant effect;
metabolism accelerated by carbamazepine, phenytoin
and possibly phenobarbital; avoid with drugs known
to cause agranulocytosis; concentration possibly
increased or decreased by valproate.
Antimalarials: avoid with artemether/lumefantrine.
Antipsychotics: avoid with depot formulations
(cannot be withdrawn quickly if neutropenia occurs);
possible increased risk of ventricular arrhythmias
with risperidone - avoid.
Antivirals: concentration increased by ritonavir -
avoid; increased risk of ventricular arrhythmias with
saquinavir - avoid.
Anxiolytics and hypnotics: increased sedative effects;
adverse reports with clozapine and benzodiazepines.
Atomoxetine: increased risk of ventricular
arrhythmias.
Cytotoxics: increased risk of agranulocytosis - avoid;
increased risk of ventricular arrhythmias with arsenic
trioxide.
Lithium: increased risk of extrapyramidal side effects
and possibly neurotoxicity.
Penicillamine: increased risk of agranulocytosis -
avoid.
Ulcer-healing drugs: effects possibly enhanced
by cimetidine; concentration possibly reduced by
omeprazole.
Metabolism
Clozapine is almost completely metabolized prior to excretion, and only trace amounts of unchanged drug are detected in the urine and feces. Clozapine is a substrate for many cytochrome P450 isozymes, in particular CYP1A2, CYP2D6, and CYP3A4.The unmethylated, hydroxylated, and N-oxide derivatives are components in both urine and feces. Pharmacological testing has shown the desmethyl metabolite (norclozapine) to have only limited activity, while the hydroxylated and N-oxide derivatives were inactive.
Metabolism
Clozapine is orally active and metabolized mainly by CYP3A4 to inactive desmethyl, hydroxyl, and N-oxide derivatives, with a half-life of approximately 12 hours. Clozapine has relatively low affinity for brain dopamine D1 and D2 receptors (moderate affinity for D4) in comparison to its affinity at adrenergic α1 and α2, histamine H1, muscarinic M1 and serotonin 5-HT2A receptors.
storage
Room temperature
References
1) Seeman and Van Tol (1994),?Dopamine receptor pharmacology;? Trends Pharmacol. Sci.,?15?264 2) Ellenbroek?et al. (1991),?The involvement of dopamine D1 and D2 receptors in the effects of the classical neuroleptic haloperidol and the atypical neuroleptic clozapine;? Eur. J. Pharmacol.,?196?103 3) Canton?et al.?(1990),?Binding of the typical and atypical antipsychotics to 5-HT1C and 5-HT2 sites: clozapine potently interacts with 5-HT1C sites;? Eur. J. Pharmacol.,?191?93 4) Kuoppamaki?et al.?(1993),?Clozapine and N-desmethylclozapine are potent 5-HT1C receptor antagonists;? Eur. J. Pharmacol., 245?179
Properties of Clozapine
| Melting point: | 182-185°C |
| Boiling point: | 482.71°C (rough estimate) |
| Density | 1.1327 (rough estimate) |
| refractive index | 1.6110 (estimate) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | ethanol: 1 mg/mL |
| form | powder |
| pka | 3.70, 7.60(at 25℃) |
| color | pale yellow |
| Merck | 14,2423 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 5786-21-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Clozapine(5786-21-0) |
Safety information for Clozapine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H341:Germ cell mutagenicity H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Clozapine
Clozapine manufacturer
Global Calcium Pvt Ltd
Nivedita Chemicals Pvt Ltd (Anek Prayog Pvt Ltd)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Clozapine 99%View Details
Clozapine 99%View Details -
 Clozapine 5786-21-0 98%View Details
Clozapine 5786-21-0 98%View Details
5786-21-0 -
 5786-21-0 98%View Details
5786-21-0 98%View Details
5786-21-0 -
 5786-21-0 Clozapine 98%View Details
5786-21-0 Clozapine 98%View Details
5786-21-0 -
 Clozapine CAS 5786-21-0View Details
Clozapine CAS 5786-21-0View Details
5786-21-0 -
 Clozapine 99%View Details
Clozapine 99%View Details
5786-21-0 -
 Clozapine CAS 5786-21-0View Details
Clozapine CAS 5786-21-0View Details
5786-21-0 -
 Clozapine Resolution Mixture CAS 5786-21-0View Details
Clozapine Resolution Mixture CAS 5786-21-0View Details
5786-21-0