Clonazepam
Synonym(s):5-(2-Chlorophenyl)-7-nitro-3H-1,4-benzodiazepin-2(1H)-one
- CAS NO.:1622-61-3
- Empirical Formula: C15H10ClN3O3
- Molecular Weight: 315.71
- MDL number: MFCD00057746
- EINECS: 216-596-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-20 16:56:11
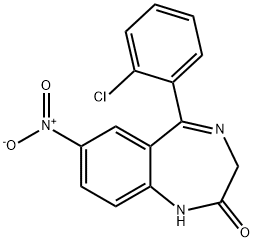
What is Clonazepam?
Absorption
Clonazepam is rapidly and almost entirely absorbed after oral administration as tablets . Peak plasma concentrations of clonazepam administered by the oral route are reached within 1-4 hours and the associated absorption half-life is about 25 minutes . The absolute bioavailability is approximately 90% - but with substantially large differences between individuals .
Toxicity
Benzodiazepines like clonazepam commonly cause drowsiness, ataxia, dysarthria, and nystagmus. Overdose with clonazepam is generally not life-threatening if the drug is taken alone, but may lead to areflexia, apnea, hypotension, cardiorespiratory depression, and coma. Coma, if it does occur, usually lasts a few hours but it can become more protracted and cyclical, especially in elderly patients. Increased frequency of seizures may occur in patients at supratherapeutic plasma concentrations. Benzodiazepine respiratory depressant effects are more serious when compounded in patients with respiratory disease.
An increased risk of congenital malformations associated with the use of benzodiazepine drugs like clonazepam has been suggested in several studies . There may also be non-teratogenic risks associated with the use of benzodiazepines during pregnancy . There have been reports of neonatal flaccidity, respiratory and feeding difficulties, and hypothermia in children born to mothers who have been receiving benzodiazepines late in pregnancy . In addition, children born to mothers receiving benzodiazepines late in pregnancy may be at some risk of experiencing withdrawal symptoms during the postnatal period . In general, it is best for patients who are of childbearing potential and also use benzodiazepines like clonazepam to discuss such matters with their health care professionals as careful consideration must be undertaken regarding the intersection of the risks of untreated seizure potential in the patient and any possible toxicity to the fetus .
Although the active ingredient of clonazepam has been found to pass into the maternal milk in small amounts only, mothers receiving clonazepam should not breast-feed their infants .
Since the possibility that adverse effects on the physical or mental development of the child could become apparent only after a number of years, the risk-benefit consideration of the long-term use of clonazepam in pediatric patients younger than five years of age is important .
The pharmacological effects of benzodiazepines like clonazepam appear to be greater in elderly patients than in younger patients even at similar plasma benzodiazepine concentrations, possibly because of age-related changes in drug-receptor interactions, post-receptor mechanisms, and organ function . In general elderly patients should be started on the lowest possible dose of clonazepam and observed closely . There is an increased risk for falls and fractures among elderly and debilitated benzodiazepine users . The risk is increased in those taking concomitant sedatives, including substances like benzodiazepines, alcoholic beverages, and so on .
Some oral LD50 values documented are >4000 mg/kg for the mouse model, >4000 mg/kg for the adult rat model, and >2000 mg/kg for the rabbit model .
Description
Clonazepam (Item No. 14263) is an analytical reference material categorized as a benzodiazepine. Clonazepam is regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications.
Description
Clonazepam is a benzodiazepine-class medication whose synthesis was reported in 1962 by Leo H. Sternbach and co-workers at Hoffmann–LaRoche (Nutley, NJ).1 The report was the tenth in a series on quinazolines and 1,4-benzodiazepines. Sternbach was a pioneer in the development of the psychotherapeutic benzodiazepines, the best known of which is former Molecule of the Week diazepam2 (Valium).
Clonazepam was originally developed as an anticonvulsant; but it is more frequently used to treat anxiety, seizures (including those associated with epilepsy), panic disorder, and some psychoses. Although the drug is considered to be nonhazardous, the US Drug Enforcement Administration classifies it as a Schedule IV controlled substance because abusing it can lead to psychological dependence.
Sternbach’s clonazepam synthesis ended with nitration of the previously unsubstituted benzene ring. The current manufacturing method, according to R. S. Vardanyan and V. J. Hruby in Synthesis of Essential Drugs, is similar to Sternbach’s synthesis: five steps starting from 2-chloro-2′-nitrobenzophenone and ending with the same nitration step.
Chemical properties
Tan Solid
Originator
Rivotril,Roche,France,1973
The Uses of Clonazepam
Antiepileptic agent with anxiolytic and antimanic properties. Anticonvulsant. This is a controlled substance (depressant)
Indications
Clonazepam is indicated as monotherapy or as an adjunct in the treatment of Lennox-Gastaut syndrome (petit mal variant), akinetic, and myoclonic seizures. Furthermore, clonazepam may also be of some value in patients with absence spells (petit mal) who have failed to respond to succinimides. Additionally, clonazepam is also indicated for the treatment of panic disorder, with or without agoraphobia, as defined in the DSM-V.
Alternatively, some regional prescribing information note that clonazepam is indicated for all clinical forms of epileptic disease and seizures in adults, especially absence seizures (petit mal) including atypical absence; primary or secondarily generalised tonic-clonic (grand mal), tonic or clonic seizures; partial (focal) seizures with elementary or complex symptomatology; various forms of myoclonic seizures, myoclonus and associated abnormal movements. Such regional label data also has clonazepam indicated for most types of epilepsy in infants and children, especially absences (petit mal), myoclonic seizures and tonic-clonic fits, whether due to primary generalized epilepsy or to secondary generalization of partial epilepsy.
Background
A benzodiazepine used to treat various seizures, including myotonic or atonic seizures, photosensitive epilepsy, and absence seizures, although tolerance may develop. The agent has also been indicated for treating panic disorder. The mechanism of action appears to involve the enhancement of gamma-aminobutyric acid receptor responses.
Since being first patented in 1960 and then released for sale from Roche in the US in 1975, clonazepam has experienced a storied history in the treatment of the aforementioned medical conditions. Now available as a generic medication, the agent continues to see exceptionally high use as millions of prescriptions are written for the medication internationally every year. Unfortunately, however, like most benzodiazepines, clonazepam use has also been associated with recreational use and drug abuse.
Definition
ChEBI: 1,3-Dihydro-2H-1,4-benzodiazepin-2-one in which the hydrogens at positions 5 and 7 are substituted by 2-chlorophenyl and nitro groups, respectively. It is used in the treatment of all types of epilepsy and seizures, as well as myoclonus nd associated abnormal movements, and panic disorders. However, its use can be limited by the development of tolerance and by sedation.
Manufacturing Process
The following description is taken from US Patent 3,116,203. A stirred solution
of 75 g of 2-amino-2'-nitrobenzophenone in 700 ml of hot concentrated
hydrochloric acid was cooled to 0°C and a solution of 21.5 g of sodium nitrite
in 50 ml of water was added in the course of 3 hours. The temperature of the
suspension was kept at 2° to 7°C during the addition. The resulting clear
solution was poured into a stirred solution of 37 g of cuprous chloride in 350
ml of hydrochloric acid 1:1. The solid which had formed after a few minutes
was filtered off, washed with water and recrystallized from ethanol. Crystals of
2-chloro-2'-nitrobenzophenone melting at 76° to 79°C were obtained.
A solution of 20 g of 2-chloro-2'-nitrobenzophenone in 450 ml of ethanol was
hydrogenated at normal pressure and room temperature with Raney nickel.
After uptake of about 6 liters of hydrogen the catalyst was filtered off, and the
alcohol then removed in vacuo. The residue was distilled in a bulb tube at 0.4
mm and a bath temperature of 150° to 165°C giving a yellow oil. The oil was
dissolved in alcohol, and on addition of water, needles of 2-amino-2'-
chlorobenzophenone melting at 58° to 60°C were obtained.
To a solution of 42 g of 2-amino-2'-chlorobenzophenone in 500 ml of benzene,
19 ml of bromoacetyl bromide was added dropwise. After refluxing for 2
hours, the solution was cooled, washed with 2 N sodium hydroxide and
evaporated. The residue was recrystallized from methanol giving crystals of 2-
bromo-2'-(2-chlorobenzoyl)acetanilide melting at 119° to 121°C.
To a solution of 14.5 g of 2-bromo-2'-(2-chlorobenzoyl)acetanilide in 100 ml
of tetrahydrofuran, an excess of liquid ammonia (ca 150 ml) was added. The
ammonia was kept refluxing with a dry-ice condenser for 3 hours after which
time the ammonia was allowed to evaporate and the solution was poured into
water. Crystals of 2-amino-2'-(2-chlorobenzoyl)acetanilide were collected,
which after recrystallization from ethanol melted at 162° to 164°C.
A solution of 3 g of 2-amino-2'-(2-chlorobenzoyl)acetanilide in 50 ml of
pyridine was refluxed for 24 hours after which time the pyridine was removed
in vacuo. The residue was recrystallized from methanol and a mixture of
dichloromethane and ether giving crystals of 5-(2-chlorophenyl)-3H-1,4-
benzodiazepin-2(1H)-one melting at 212° to 213°C.
To a solution of 13.5 g of 5-(2-chlorophenyl)-3H-1,4-benzodiazepin-2(1H)-one
in 60 ml of concentrated sulfuric acid, a solution of 5.5 g of potassium nitrate
in 20 ml concentrated sulfuric acid was added dropwise. The solution then was
heated in a bath at 45° to 50°C for 2.5 hours, cooled and poured on ice. After
neutralizing with ammonia, the formed precipitate was filtered off and boiled
with ethanol. A small amount of white insoluble material was then filtered off.
The alcoholic solution on concentration yielded crystals of 7-nitro-5-(2-
chlorophenyl)-3H-1,4-benzodiazepin-2(1H)-one which, after recrystallization
from dichloromethane, melted at 238° to 240°C.
brand name
Klonopin (Roche). Antelepsin (Arzneimittelwerk Dresden), Clonopin/ Rivotril (Roche).
Therapeutic Function
Anticonvulsant
General Description
Clonazepam is useful in absence seizures and in myoclonicseizures. Tolerance to the anticonvulsant effect of the clonazepamoften developed rather quickly, and it is a commonproblem with the BZDs. Metabolism involves hydroxylationof the C-3 position, followed by glucuronidation andnitro group reduction, followed by acetylation.
Hazard
Moderately toxic.
Pharmacokinetics
The pharmacodynamic properties of clonazepam are common among benzodiazepines and include anticonvulsive, sedative, muscle relaxing and anxiolytic effects . Animal data and electroencephalographic investigations in man have shown that clonazepam rapidly suppresses many types of paroxysmal activity including the spike and wave discharge in absence seizures (petit mal), slow spike wave, generalized spike wave, spikes with temporal or other locations, as well as irregular spikes and waves . Moreover, the agent can also decrease the frequency, amplitude, duration, and spread of discharge in minor motor seizures .
Generalized EEG abnormalities are more readily suppressed by clonazepam than are focal EEG abnormalities such as focal spikes . Clonazepam has beneficial effects in generalized and focal epilepsies .
Clinical Use
Benzodiazepine:
Anticonvulsant
Anxiolytic
Restless leg syndrome
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism possibly increased by
rifampicin.
Antipsychotics: increased sedative effects; increased
risk of hypotension, bradycardia and respiratory
depression with parenteral clonazepam and IM
olanzapine; risk of serious adverse effects in
combination with clozapine.
Antivirals: concentration possibly increased by
ritonavir.
Disulfiram: metabolism inhibited, increased sedative
effects.
Sodium oxybate: enhanced effects of sodium oxybate
- avoid.
Metabolism
Clonazepam is metabolized principally in the liver . The metabolic pathways include hydroxylation, reduction of the nitro groups to amine groups, and the addition of acetate to the amino grouping . In particular, clonazepam is extensively metabolized by reduction to 7-amino-clonazepam and by N-acetylation to 7-acetamido-clonazepam . Hydroxylation at the C-3 position also occurs . Hepatic cytochrome P450 3A4 is implicated in the nitroreduction of clonazepam to pharmacologically inactive metabolites .
Metabolism
Clonazepam is extensively metabolised in the liver, its principal metabolite being 7-aminoclonazepam, which has no antiepileptic activity; minor metabolites are the 7-acetamido- and 3-hydroxy-derivatives. It is excreted mainly in the urine almost entirely as its metabolites in free or conjugated form.
Properties of Clonazepam
| Melting point: | 236.5-238.50C |
| Boiling point: | 524.5±50.0 °C(Predicted) |
| Density | 1.4592 (rough estimate) |
| refractive index | 1.6470 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, slightly soluble in alcohol and in methanol. |
| appearance | white crystals or powder |
| form | powder |
| pka | 1.5, 10.5(at 25℃) |
| color | light yellow |
| CAS DataBase Reference | 1622-61-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Clonazepam(1622-61-3) |
| EPA Substance Registry System | 2H-1,4-Benzodiazepin-2-one, 5-(2-chlorophenyl)-1,3-dihydro-7-nitro- (1622-61-3) |
Safety information for Clonazepam
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Clonazepam
Clonazepam manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
