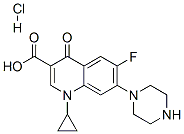
Ciprofloxacin hydrochloride
Synonym(s):Ciprofloxacin hydrochloride monohydrate
- CAS NO.:93107-08-5
- Empirical Formula: C17H18FN3O3.HCl
- Molecular Weight: 367.8
- EINECS: 642-985-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Ciprofloxacin hydrochloride?
The Uses of Ciprofloxacin hydrochloride
Anti-bacterial
The Uses of Ciprofloxacin hydrochloride
Ciprofloxacin hydrochloride is a fluoroquinolone antibiotic that is widely used as an antimicrobial and immunomodulatory agent. Its range of activity includes most strains of bacterial pathogens capable of inducing respiratory, urinary tract, gastrointestinal, and abdominal infections. It functions by inhibiting DNA gyrase (a type II topoisomerase) and topoisomerase IV, the enzymes responsible for negative supercoiling of chromosomes and DNA strand separation, thus blocking initiation of bacterial replication. Most recently Ciprofloxacin hydrochloride has been used in the management of postexposure inhalational anthrax and radiation combined injury resulting from nuclear disasters.[Cayman Chemical]
What are the applications of Application
Ciprofloxacin HCl is a fluoroquinolone DNA gyrase/topoisomerase-targeting antibiotic often used in cell culture.
Definition
ChEBI: Ciprofloxacin hydrochloride (anhydrous) is the anhydrous form of the monohydrochloride salt of ciprofloxacin. It has a role as an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor, an antibacterial drug, a topoisomerase IV inhibitor and an antiinfective agent. It contains a ciprofloxacin.
brand name
Ciloxan (Alcon); Cipro (Bayer); Proquin (Esprit).
Biological Activity
ciprofloxacin (hydrochloride) is a fluoroquinolone antibiotic that has antimicrobial and immunomodulatory activities [1].ciprofloxacin functions by inhibiting dna gyrase and topoisomerase iv, the enzymes responsible for negative supercoiling of chromosomes and dna strand separation, thus blocking initiation of bacterial replication. topoisomerase iv is the primary target of ciprofloxacin in s. aureus [2].ciprofloxacin (hydrochloride) is a fluoroquinolone antibiotic that acts as an antimicrobial and immunomodulatory agent. in b6d2f1/j mice received radiation combined injury (ci), ciprofloxacin significantly reduced pro-inflammatory cytokine and chemokine concentrations (il-6 and kc), and enhanced il-3 production. cip also inhibited ci-induced apoptosis and autophagy in ileal villi, systemic bacterial infection, and iga production [1]. ciprofloxacin (hydrochloride) had been approved by fda for management of postexposure inhalational anthrax. in animals after exposure to aerosolized b. anthracis, ciprofloxacin significantly improved survival rate. ciprofloxacin inhibited the growth of b. anthracis with mic90 of 0.06 μg/ml. in the usamriid rhesus monkey model of inhalational anthrax, the maximum concentration (cmax) of ciprofloxacin was 1.74 μg/ml and the minimum concentration (cmin) was 0.17 μg/ml [3].
Synthesis
The preparation method of ciprofloxacin HCl, its step is as follows:
a) cyclopropyl carboxylic acid is added in the organic solvent, and the adding piperazine, reacted 3~8 hours down as acid binding agent with alkali at 60~120 ℃, the monitoring cyclopropyl carboxylic acid is residual less than 2% o'clock termination reaction, stirring is cooled to 0~30 ℃, leaves standstill suction filtration;
b) add water and hydrochloric acid in the products therefrom toward a) step, under agitation be warming up to molten clearly, add activated carbon decolorizing, filtered while hot, and transfer filtrate pH to 2~4 with hydrochloric acid, and the cooling crystallization filters, rinsing, and drying makes ciprofloxacin HCl.
References
[1]. fukumoto r, cary lh, gorbunov nv, et al. ciprofloxacin modulates cytokine/chemokine profile in serum, improves bone marrow repopulation, and limits apoptosis and autophagy in ileum after whole body ionizing irradiation combined with skin-wound trauma. plos one. 2013;8(3):e58389.
[2]. drlica k, zhao x. dna gyrase, topoisomerase iv, and the 4-quinolones. microbiol mol biol rev. 1997 sep;61(3):377-92.
[3]. meyerhoff a, albrecht r, meyer jm, et al. us food and drug administration approval of ciprofloxacin hydrochloride for management of postexposure inhalational anthrax. clin infect dis. 2004 aug 1;39(3):303-8.
Properties of Ciprofloxacin hydrochloride
| Melting point: | >300 ºC |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | insoluble in EtOH; ≥33.87 mg/mL in H2O; ≥9.34 mg/mL in DMSO with ultrasonic |
| form | crystalline solid |
| color | White to off-white |
| InChI | InChI=1S/C17H18FN3O3.ClH/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24;/h7-10,19H,1-6H2,(H,23,24);1H |
| CAS DataBase Reference | 93107-08-5 |
Safety information for Ciprofloxacin hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ciprofloxacin hydrochloride
| InChIKey | DIOIOSKKIYDRIQ-UHFFFAOYSA-N |
| SMILES | O=C(O)C1C(=O)C2=CC(=C(C=C2N(C2CC2)C=1)N1CCNCC1)F.[H]Cl |
Ciprofloxacin hydrochloride manufacturer
Attar Global
B. Shah & Sons
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Ciprofloxacin hydrochloride 98%View Details
Ciprofloxacin hydrochloride 98%View Details -
 Ciprofloxacin HCL, 25KgView Details
Ciprofloxacin HCL, 25KgView Details
86393-32-0 -
 Ciprofloxacin Hydrochloride API PowderView Details
Ciprofloxacin Hydrochloride API PowderView Details
86393-32-0 -
 Ciprofloxacin Hydrochloride Powder, 99%View Details
Ciprofloxacin Hydrochloride Powder, 99%View Details
86393-32-0 -
 Ciprofloxacin HCL PowderView Details
Ciprofloxacin HCL PowderView Details
86393-32-0 -
 Ciprofloxacin Hydrochloride Powder, BPView Details
Ciprofloxacin Hydrochloride Powder, BPView Details
86393-32-0 -
 50Kg Ciprofloxacin Hydrochloride PowderView Details
50Kg Ciprofloxacin Hydrochloride PowderView Details
86393-32-0 -
 Ciprofloxacin HCl, 25KgView Details
Ciprofloxacin HCl, 25KgView Details
86393-32-0
