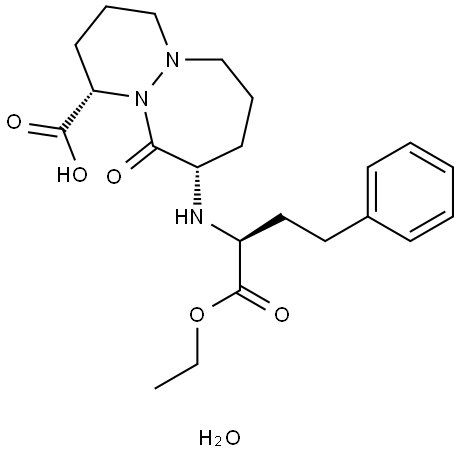
Cilazapril
Synonym(s):Cilastatin sodium;Cilazapril monohydrate
- CAS NO.:88768-40-5
- Empirical Formula: C22H31N3O5
- Molecular Weight: 417.5
- MDL number: MFCD00865788
- EINECS: 641-006-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:30:59
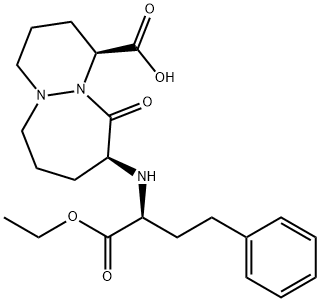
What is Cilazapril?
Absorption
Maximum plasma concentrations of cilazaprilat are reached within two hours after administration of cilazapril.
Toxicity
Limited data are available with regard to overdosage in humans. The most likely manifestations are hypotension, which may be severe, hyperkalaemia, hyponatraemia and renal impairment with metabolic acidosis. Treatment should be mainly symptomatic and supportive.
Description
Cilazapril is a prodrug form of the angiotensin converting enzyme (ACE) inhibitor cilazaprilat. In vivo, cilazapril (0.1 mg/kg) inhibits plasma ACE activity and inhibits the angiotensin I-induced pressor response in anesthetized rats and cats. It decreases systolic blood pressure in spontaneously hypertensive rats when administered at a dose of 30 mg/kg. Cilazapril (10 mg/kg, p.o.) decreases blood pressure in volume-depleted renal hypertensive dogs. Formulations containing cilazapril have been used in the treatment of hypertension.
Chemical properties
Off-White Solid
The Uses of Cilazapril
An ACE inhibitor. Hydrolyzed in vivo to the active diacid metabolite (Cilazaprilat). Antihypertensive
The Uses of Cilazapril
An ACE inhibitor. Hydrolyzed in vivo to the active diacid metabolite (Cilazaprilat). Antihypertensive.
Indications
Cilazapril is an ACE inhibtor class drug used in the treatment of hypertension and heart failure.
Background
Cilazapril is an ACE inhibtor class drug used in the treatment of hypertension and heart failure. It belongs to the angiotensin-converting enzyme inhibitors (ACE inhibitors) class of drugs. It is a prodrug that is hydrolyzed after absorption to its main metabolite cilazaprilat. It is branded as Inhibace in Canada and other countries, Vascace and Dynorm in a number of European countries, among many other names. None of these varieties are available in the United States.
What are the applications of Application
Cilazapril is a potent ACE inhibitor
Definition
ChEBI: Cilazapril is a pyridazinodiazepine resulting from the formal condensation of the carboxy group of cilazaprilat with ethanol. It is a drug used in the treatment of hypertension and heart failure. It has a role as a prodrug, an antihypertensive agent and an EC 3.4.15.1 (peptidyl-dipeptidase A) inhibitor. It is an ethyl ester, a pyridazinodiazepine and a dicarboxylic acid monoester. It is functionally related to a Cilazaprilat.
brand name
Inhibace (Hoffmann-LaRoche).
Pharmacokinetics
Cilazapril inhibits the production angiotensin II. By doing so, it decreases sodium and water reabsorption (via aldosterone) and it decreases vasoconstriction. The combined effect of this is a decrease in vascular resistance, and therefore, blood pressure. The absolute bioavailability of cilazaprilat after oral administration of cilazapril is 57% based on urinary recovery data. (The absolute bioavailability of cilazaprilat after oral administration of cilazaprilat is 19%.) Ingestion of food immediately before the administration of cilazapril reduces the average peak plasma concentration of cilazaprilat by 29%, delays the peak by one hour and reduces the bioavailability of cilazaprilat by 14%. These pharmacokinetic changes have little influence on plasma ACE inhibition.
Metabolism
Properties of Cilazapril
| Melting point: | 95-97°C |
| Boiling point: | 598.1±60.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethanol (Slightly, Sonicated), Methanol (Slightly) |
| form | powder |
| pka | 2.28±0.20(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 88768-40-5(CAS DataBase Reference) |
Safety information for Cilazapril
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cilazapril
Cilazapril manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran

You may like
-
 88768-40-5 Cilazapril 98%View Details
88768-40-5 Cilazapril 98%View Details
88768-40-5 -
 88768-40-5 98%View Details
88768-40-5 98%View Details
88768-40-5 -
 Cilazapril 98%View Details
Cilazapril 98%View Details
88768-40-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8