CHROMOMYCIN A3
Synonym(s):Aburamycin B;Chromomycin A₃ - CAS 7059-24-7 - Calbiochem;CMA3;NSC 58514;Toyomycin
- CAS NO.:7059-24-7
- Empirical Formula: C57H82O26
- Molecular Weight: 1183.25
- MDL number: MFCD00043151
- EINECS: 230-348-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
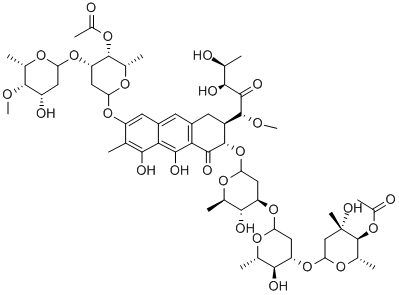
What is CHROMOMYCIN A3?
Description
Chromomycin A3 is an anthraquinone antibiotic and antitumor agent isolated from S. griseus that is used as a fluorescent probe for DNA with excitation/emission spectra of 445/575 nm. Its DNA binding is specific to two or more contiguous GC base pairs, which makes it suitable for characterizing heterochromatin in plants with species-specific AT:GC ratios. Chromomycin A3 is cytotoxic against non-small cell lung cancer and cervical cancer in vitro (IC50s = 1, 42, 60, and 40 nM for HCC44, A549, ME180, and HeLa cells, respectively). It also inhibits oxidative stress- and DNA damage-induced neuronal injury by enhancing Sp1 and Sp3 transcription factor binding.
Chemical properties
Yellow powder
The Uses of CHROMOMYCIN A3
Chromomycin A3 is the major component of the chromomycin complex of the aureolic acid class, isolated from several Streptomyces species, and first reported in 1960. Chromomycin A3 exhibits a broad biological profile as an antibacterial, antifungal and antitumour agent. It binds reversibly to GC-specific DNA ligand in the minor groove which inhibits transcription, DNA gyrase and topoisomerase II activity. The intense UV spectrum and strong fluorescence makes chromomycin a useful stain for DNA.
The Uses of CHROMOMYCIN A3
Fluorescent DNA stain in flow cytometry and karyotype analysis of chromosomes.
The Uses of CHROMOMYCIN A3
Chromomycin A3, is used as a G-C specific DNA ligand which inhibits transcription and acts as a DNA binding dye. Chromomycin A3 antagonizes enhanced DNA binding of the transcription factors Sp1 and Sp3 to their cognate "G-C" box, induced by oxidative stress or DNA damage. Furthermore, by inhibiting transcription, Chromomycin A3 and its structural analogs can inhibit protein biosynthesis.
What are the applications of Application
Chromomycin A3 is a G-C specific DNA ligand which inhibits transcription and acts as a DNA binding dye
Definition
ChEBI: Chromomycin A3 is a chromomycin.
General Description
Chemical structure: aureolic acid
Biochem/physiol Actions
Chromomycin A3 from Streptomyces griseus is an antibiotic exhibiting anti-bacterial, anti-fungal and antitumor activities. It serves as a fluorescent DNA stain. It is useful for the detection of protamine deficiency in sperm chromatin. The compound blocks macromolecule synthesis by a specific, reversible interaction with DNA in the presence of bivalent metal ions. Binding to DNA minor groove mediates an efficient competitive inhibition of DNA gyrase and significantly affects topoisomerase II activity.
Purification Methods
Dissolve the antibiotic (10g) in EtOAc and add to a column of Silica Gel (Merck 0.05-0.2microns, 4x70cm) in EtOAc containing 1% oxalic acid. Elute with EtOAc+1% oxalic acid and check fractions by TLC. Pool fractions, wash with H2O thoroughly, dry and evaporate. Recrystallise the residue from EtOAc. The heptaacetate has m 214o, [] D -20o (c 1, EtOH). [Miyamoto et al. Tetrahedron 23 421 1967, Harada et al. J Am Chem Soc 91 5896 1969, Beilstein 17/5 V 673.]
References
Van Dyke et al. (1983), Chromomycin, Mithramycin and olivomycin binding sites on heterogeneous deoxyribonucleic acid. Footprinting with methidiumpropyl-EDTA)iron(II); ?Biochemistry, 22 2373 Crissman and Tobey (1990), Specific staining of DNA with the fluorescent antibiotic, mithramycin, chromomycin, and olivomycin; Methods Cell Biol., 33 97 Chatterjee et al. (2001), Sequence-selective DNA binding drugs mithramycin A and chromomycin A3 are potent inhibitors of neuronal apoptosis induced by oxidative stress and DNA damage in cortical neurons; Neurol., 49 345 Miller et al. (2010), Identification of known drugs that act as inhibitors of NF-kappaB signaling and their mechanism of action; Pharmacol., 79 1272 Dutta et al. (2020), Comparative analysis of tests used to assess sperm chromatin integrity and DNA fragmentation; Andrologia, 53?e13718
Properties of CHROMOMYCIN A3
| Melting point: | 185℃ |
| Boiling point: | 780.13°C (rough estimate) |
| alpha | D23 -57° (ethanol) |
| Density | 1.1451 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol, DMSO, and ethyl acetate (10 mg/ml). |
| pka | 4.54±0.60(Predicted) |
| form | Yellow solid |
| color | Yellow |
| Merck | 13,2258 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
Safety information for CHROMOMYCIN A3
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for CHROMOMYCIN A3
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran

You may like
-
 Chromomycin A3 from Streptomyces griseus CAS 7059-24-7View Details
Chromomycin A3 from Streptomyces griseus CAS 7059-24-7View Details
7059-24-7 -
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3