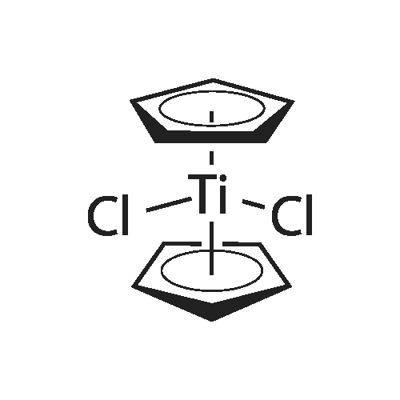
Chromocene
Synonym(s):Chromocene;Di(cyclopentadienyl)chromium(II)
- CAS NO.:1271-24-5
- Empirical Formula: C10H10Cr10*
- Molecular Weight: 182.18
- MDL number: MFCD00013750
- EINECS: 215-036-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
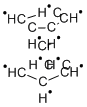
What is Chromocene?
Chemical properties
bordeaux crystalline powder
The Uses of Chromocene
Provides catalyst for ethylene polymerization. A precursor to other chromium(II) compounds.
What are the applications of Application
Bis(cyclopentadienyl)chromium(II) is a catalyst for stereoselective pinacol-type cross-coupling reactions
General Description
Red needles or reddish-purple solid.
Air & Water Reactions
Chromocene is very sensitive to exposure to air and moisture (decomposition is not vigorous). Decomposes in water .
Reactivity Profile
Chromocene reacts with water and alcohols. . It's reaction with alcohols produces pyrophoric metal alkoxides (dialkoxychromium and cyclopentadiene).
Fire Hazard
Literature sources indicate that Chromocene is flammable.
Purification Methods
Chromocene forms pyrophoric red crystals on sublimation at 50o/0.1mm followed by resublimation at 75-90o/0.1mm. Although it is stable at least to 300o, it is readily oxidised in air, and effervesces slowly in H2O to give cyclopentadiene. All operations should be carried out in a dry box. It decomposes in CCl4 or CS2, and for IR even grinding with nujol, KBr or KI causes some decomposition. [Wilkinson et al. J Inorg Nucl Chem 95 109 1956, Wilkinson J Am Chem Soc 76 209 1954, Beilstein 16 IV 1774.]
Properties of Chromocene
| Melting point: | 168-170 °C(lit.) |
| storage temp. | Store below +30°C. |
| form | crystal |
| color | scarlet |
| Water Solubility | Insoluble in water. |
| Sensitive | Air & Moisture Sensitive |
| NIST Chemistry Reference | Bis(«eta»5-cyclopentadienyl) chromium(1271-24-5) |
| EPA Substance Registry System | Chromocene (1271-24-5) |
Safety information for Chromocene
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chromocene
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Bis (cyclopentadienyl)chromium, Sublimed CAS 1271-24-5View Details
Bis (cyclopentadienyl)chromium, Sublimed CAS 1271-24-5View Details
1271-24-5 -
 Bis (cyclopentadienyl)chromium, Sublimed CAS 1271-24-5View Details
Bis (cyclopentadienyl)chromium, Sublimed CAS 1271-24-5View Details
1271-24-5 -
 Bis(cyclopentadienyl)chromium(II) CAS 1271-24-5View Details
Bis(cyclopentadienyl)chromium(II) CAS 1271-24-5View Details
1271-24-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1