CHROMIUM(III) NITRATE NONAHYDRATE
Synonym(s):Chromic Nitrate;Chromium nitrate nonahydrate;Chromium trinitrate nonahydrate;Chromium(III) nitrate nonahydrate
- CAS NO.:7789-02-8
- Empirical Formula: CrH18N3O18
- Molecular Weight: 400.14832
- MDL number: MFCD00149659
- EINECS: 616-540-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-11 16:58:07
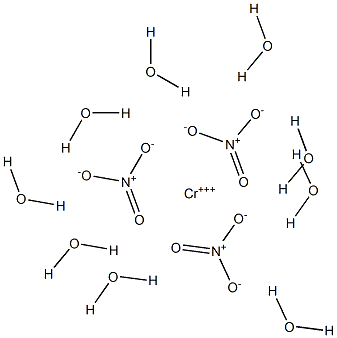
What is CHROMIUM(III) NITRATE NONAHYDRATE?
Chemical properties
deep blue or violet crystals
Chemical properties
Chromium nitrate is a crystalline substance, variously stated to be green brown or purple and existing in various hydrated forms.
The Uses of CHROMIUM(III) NITRATE NONAHYDRATE
Chromium nitrate is used in the manufacture of alkali metal-free catalysts and in preservation. It is used in the preparation of chrome catalysts, in textile printing operations, and as a corrosion inhibitor. Chromium(III) nitrate as a source of chromium is added to food supplements for nutritional purposes .
Potential Exposure
Chromium nitrate is used in the preparation of chrome catalysts, in textile printing operations; and as a corrosion inhibitor. Used to make other chemicals.
Shipping
UN2720 Chromium nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer
Purification Methods
The pure deep violet nonahydrate salt is best prepared freshly from pure recrystallised (3 times) chromium (VI) trioxide and pure nitric acid. A solution is prepared by dissolving 1g of CrO3 in 3mL of H2O and 2mL of pure HNO3, and carefully, with stirring (behind a screen) pure MeOH (0.5-1.0mL) is added dropwise carefully (shield) with cooling to avoid a violent reaction. A bluish colour develops as Cr(VI) is reduced to Cr(III). When this solution is diluted to 130,000ppm of the salt, analysis detected by ICP/MS gave the following trace elements (ppm in brackets): Sr (33), Na (3.5), Mo, Ni, Cu, Si and Mg (0.13 each), Se, Zn, Al (0.026 each), Ti (1.5), Fe (4.9), Co (0.07), Sn (0.065), Ba (0.013 and W (0.078). Evaporation of the methanolic solution in high vacuum over CaCl2 eventually yields pure deep violet rhombic crystals of chromium (III) nitrate nonahydrate. An aqueous solution of this salt becomes green on heating but reverts to the violet colour on cooling.
Incompatibilities
This chemical is a strong oxidizer. Contact with reducing agents; fuels, ethers, and other flammable and combustible materials cause a fire and explosion hazard. Violent reaction with many compounds, including reducing agents; alcohols, chemically active metals; combustible materials, strong acids, alkaline earth sulfides, aluminum carbides, aluminum, amines, calcium sulfide, carbides, chlorine trifluoride, glycerin, hydrides, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, hydroxylamine, magnesium, metal powders, metal sulfides, molybdenum, phenylhydrazine, phosphorous red/friction, phosphorous trichloride, silicon, sulfides, sulfur, sulfur Chromium Nitrate 845 dioxide, sulfur/friction, sulfuric acid, tungsten, hydrogen trisulfide
Waste Disposal
Precipitate chromium as the hydroxide. Dewater the sludge and dispose of in singlepurpose dumps.
Properties of CHROMIUM(III) NITRATE NONAHYDRATE
| Melting point: | 60 °C(lit.) |
| Boiling point: | 100°C |
| Density | 1.80 g/mL(lit.) |
| storage temp. | Store at +15°C to +25°C. |
| solubility | H2O: very soluble(lit.) |
| form | Solid |
| color | Violet to black |
| PH | 2-3 (50g/l, H2O, 20℃) |
| Water Solubility | Soluble in water and alcohol. |
| Merck | 14,2226 |
| Exposure limits | NIOSH: IDLH 25 mg/m3; TWA 0.5 mg/m3 |
| Stability: | Stable at room temperature, but may explode if heated. Incompatible with organic materials, reducing agents. Use polyethylene tools. |
| CAS DataBase Reference | 7789-02-8(CAS DataBase Reference) |
Safety information for CHROMIUM(III) NITRATE NONAHYDRATE
| Signal word | Warning |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CHROMIUM(III) NITRATE NONAHYDRATE
CHROMIUM(III) NITRATE NONAHYDRATE manufacturer
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Chromium(III) nitrate nonahydrate, nonahydrate CAS 7789-02-8View Details
Chromium(III) nitrate nonahydrate, nonahydrate CAS 7789-02-8View Details
7789-02-8 -
 Chromium(III) nitrate nonahydrate, nonahydrate CAS 7789-02-8View Details
Chromium(III) nitrate nonahydrate, nonahydrate CAS 7789-02-8View Details
7789-02-8 -
 Chromium(III) nitrate nonahydrate, nonahydrate CAS 7789-02-8View Details
Chromium(III) nitrate nonahydrate, nonahydrate CAS 7789-02-8View Details
7789-02-8 -
 Chromium (III) nitrate nonahydrate CAS 7789-02-8View Details
Chromium (III) nitrate nonahydrate CAS 7789-02-8View Details
7789-02-8 -
 Chromium (III) nitrate nonahydrate CAS 7789-02-8View Details
Chromium (III) nitrate nonahydrate CAS 7789-02-8View Details
7789-02-8 -
 2150954 99%View Details
2150954 99%View Details
2150954 -
 CHROMIUM (III) NITRATE NONAHYDRATE Extra Pure CASView Details
CHROMIUM (III) NITRATE NONAHYDRATE Extra Pure CASView Details -
 CHROMIUM (III) NITRATE NONAHYDRATE AR CASView Details
CHROMIUM (III) NITRATE NONAHYDRATE AR CASView Details