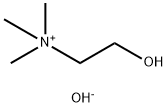
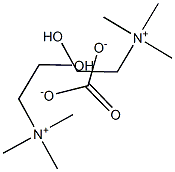
sn-Glycero-3-phosphocholine
Synonym(s):Choline alphoscerate;Choline Glycerophosphate
- CAS NO.:28319-77-9
- Empirical Formula: C8H20NO6P
- Molecular Weight: 257.22
- MDL number: MFCD00063544
- EINECS: 248-962-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-02 14:14:00
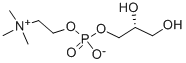
What is sn-Glycero-3-phosphocholine?
Chemical properties
Choline glycerophosphate (GPC) is a white waxy solid that is miscible with water, soluble in methanol and ethanol, and insoluble in chloroform, ether, oil, etc. It has no characteristic absorption under UV visible light. The methods for determining GPC content include high-performance liquid chromatography (equipped with an evaporative light scattering detector or refractive index differential detector) and the digestion phosphorus determination method.
The Uses of sn-Glycero-3-phosphocholine
L-α-Glycerophosphorylcholine has been used to rescue choline auxotrophy. It has also been used for the synthesis of glycerophospholipids.
Definition
ChEBI: Choline alfoscerate is a member of the class of phosphocholines that is the choline ester of sn-glycero-3-phosphate. It is one of the major osmolyte in the renal medullary cells. It has a role as a parasympatholytic, a neuroprotective agent, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a member of sn-glycerol 3-phosphates and a member of phosphocholines.
General Description
L-α-Glycerophosphorylcholine is a phospholipid, which is a derivative of phosphatidylcholine.
Biochem/physiol Actions
Increases inositol phosphate formation.
Reactivity
Choline glycerophosphate has been shown to have a high reactivity with ethanolamine, which leads to the formation of an intermediate called gamma-aminobutyric acid (GABA). This reaction occurs at a pH optimum of 5.5-7.5 and reaches its maximum rate at around pH 6.0. The optimum concentration for this reaction is 1 mM, which corresponds to an amount of 0.1 mg/mL in solution. Biological samples containing choline glycerophosphate may be analyzed using surface methodology such as atomic force microscopy or scanning electron microscopy, or by reacting with trifluoroacetic acid to form fluorescent derivatives that can be detected.
Properties of sn-Glycero-3-phosphocholine
| Melting point: | 142.5-143° |
| Boiling point: | 480℃[at 101 325 Pa] |
| alpha | D25 -2.7° (c = 2.7 in water, pH 2.5); D25 -2.8° (c = 2.6 in water, pH 5.8) |
| vapor pressure | 0Pa at 25℃ |
| Flash point: | 11 °C |
| storage temp. | -20°C |
| solubility | DMSO (Slightly, Heated, Sonicated), Methanol (Sparingly), Water (Sparingly) |
| form | solid |
| color | White to Off-White |
| Water Solubility | 1000g/L at 25℃ |
| Stability: | Very Hygroscopic |
| InChI | InChI=1/C8H20NO6P/c1-9(2,3)4-5-14-16(12,13)15-7-8(11)6-10/h8,10-11H,4-7H2,1-3H3/t8-/s3 |
| CAS DataBase Reference | 28319-77-9(CAS DataBase Reference) |
Safety information for sn-Glycero-3-phosphocholine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for sn-Glycero-3-phosphocholine
| InChIKey | SUHOQUVVVLNYQR-SBYBRXNCNA-N |
| SMILES | P([O-])(=O)(OCC[N+](C)(C)C)OC[C@H](O)CO |&1:12,r| |
sn-Glycero-3-phosphocholine manufacturer
Anthem Biosciences Pvt Ltd
Meteoric Biopharmaceuticals Pvt. Ltd.
BDR Pharmaceuticals International Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 28319-77-9 Choline alfoscerate 99%View Details
28319-77-9 Choline alfoscerate 99%View Details
28319-77-9 -
 28319-77-9 98%View Details
28319-77-9 98%View Details
28319-77-9 -
 Choline alfoscerate 98%View Details
Choline alfoscerate 98%View Details
28319-77-9 -
 Choline alfoscerate 28319-77-9 99%View Details
Choline alfoscerate 28319-77-9 99%View Details
28319-77-9 -
 Choline glycerophosphate 97% CAS 28319-77-9View Details
Choline glycerophosphate 97% CAS 28319-77-9View Details
28319-77-9 -
 Choline alfoscerate 98.00% CAS 28319-77-9View Details
Choline alfoscerate 98.00% CAS 28319-77-9View Details
28319-77-9 -
 L-α-Glycerophosphorylcholine CASView Details
L-α-Glycerophosphorylcholine CASView Details -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1