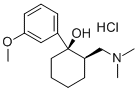
Chlorprothixene hydrochloride
Synonym(s):2-Chloro-9-(3-dimethylaminopropylidene)thioxanthene hydrochloride
- CAS NO.:6469-93-8
- Empirical Formula: C18H19Cl2NS
- Molecular Weight: 352.32
- MDL number: MFCD01941605
- EINECS: 229-289-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
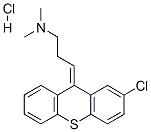
What is Chlorprothixene hydrochloride ?
Chemical properties
Pale Yellow Solid
Originator
aractan, Roche, France ,1960
The Uses of Chlorprothixene hydrochloride
Chlorprothixene hydrochloride has been used to study its exposure effects on learning and memory of rats.
The Uses of Chlorprothixene hydrochloride
Antipsychotic.
The Uses of Chlorprothixene hydrochloride
Neuroleptic;D2 dopamine receptor antagonist
What are the applications of Application
Chlorprothixene Hydrochloride is a D2DR inhibitor
Manufacturing Process
Chlorprothixene may be prepared as described in US Patent 2,951,082.
Magnesium turnings, 4.86 g (0.2 g-atom) was placed in a 500 ml reaction
flask fitted with a mercury sealed stirrer, reflux condenser and a dropping
funnel. Tetrahydrofuran, 50 ml and calcium hydride, 500 mg, were added.
Ethyl bromide, 2.18 g and a crystal of iodine then were added. A vigorous
reaction set in that evolved sufficient heat to induce refluxing. After 5
minutes, a solution of 3-dimethylaminopropyl chloride (dried over calcium
hydride) in 50 ml of tetrahydrofuran was added to the refluxing solution at
such a rate that gentle refluxing was maintained. The addition required 25
minutes.
The reaction mixture was stirred at reflux for an additional 30 minutes when
nearly all of the magnesium had dissolved and determination of magnesium in
an aliquot of the solution showed that an 82% yield of Grignard reagent had
been obtained. The reaction mixture was cooled in an ice bath and stirred
while 24.67 g (0.1 mol) of 2-chlorothiaxanthone was added over a period of
10 minutes. The reaction was stirred at room temperature for 30 minutes
then allowed to stand overnight in the refrigerator. The tetrahydrofuran was
evaporated at 50°C under reduced pressure. Benzene, 150 ml, was added to
the residue.
The mixture was hydrolyzed in the cold by the dropwise addition of 50 ml of
water. The benzene layer was separated by decantation and the gelatinous
precipitate washed with two 100 ml portions of benzene.
The precipitate was then mixed with diatomaceous earth, collected on a filter,
and washed with water and extracted with two 100 ml portions of boiling
benzene. The aqueous filtrate was extracted with 50 ml of benzene, the
combined benzene extracts washed with water and evaporated to dryness
under reduced pressure. The crystalline residue, MP 140° to 147°C, weighed
30.8 g. Recrystallization from a mixture of benzene and hexane gave 27.6 g
(83%) of 2-chloro-10-(3-dimethylaminopropyl)-10-hydroxythiaxanthene, MP
152° to 154°C. Analytically pure material from another experiment melted at
153° to 154°C.
2-Chloro-10-(3-dimethylaminopropyl)-10-hydroxythiaxanthene, 3.34 g (0.01
mol) obtained as described was dissolved in 15 ml of dry, alcohol-free
chloroform. Acetyl chloride, 2.36 g (0.03 mol) was added and the clear yellow
solution was refluxed for one hour in a system protected by a drying tube.
The solvent then was evaporated on the steam bath under reduced pressure
and the residue dissolved in absolute alcohol. The hydrochloride of 2-chloro-
10-(3-dimethylaminopropylidene)-thiaxanthene was precipitated by the
cautious addition of absolute ether. After drying at 70°C the yield of white
crystalline 2-chloro10-(3-dimethylaminopropylidene)-thiaxanthene
hydrochloride, MP 189 to 190°C (to a cloudy melt), was 3.20 g (90%). This
material is a mixture of geometric isomers.
Trans-2-chloro-9-(ω-dimethylamino-propylidene)-thioxanthene [MP 98°C, MP
of the hydrochloride 225°C (corr.)], is a valuable medicinal agent, being used
as a tranquilizer and antiemetic agent, whereas the corresponding cis isomer
(MP 44°C, MP of the hydrochloride 209°C) is not useful for these indications,
as described in US Patent 3,115,502, which describes procedures for
conversion of the cis to the trans form.
Therapeutic Function
Tranquilizer
General Description
Chlorprothixene is a neuroleptic drug, which belongs to thioxanthene group. It has anticholinergic effects. Chlorprothixene might be associated with obstructive jaundice and parkinsonism. It is used to treat psychosis.
Biological Activity
chlorprothixene (hydrochloride) is an antagonist of dopamine receptor and histamine receptors [1]. chlorprothixene is also an inhibitor of gabaa receptor [2]. all of these three receptors are implicated in many neurological processes, including motivation, pleasure, cognition, memory and learning.
in vitro
chlorprothixene exihibited strong binding affinities to dopamine and histamine receptors, the ki values of d1, d2, d3, d5 and h1 were 18nm, 2.96 nm, 4.56 nm, 9 nm and 3.75 nm, respectively. chlorprothixene showed little affinity to h3 with the ki value of >1000 nm[1]. in cos-7 cells transiently expressed rat 5-ht7 receptors and hek-293 cells stably transfected with rat 5-ht6, the ki values of chlorprothixene were 5.6 nm and 3 nm, respectively [3]. in vero 76 cells, chlorprothixene treatment inhibited sars-cov replication, with ic50 of 16.7 μm for urbani strain, 13.0 μm for frankfurt-1, 18.5 μm for chuk-w1 and 15.8 μm for toronto-2 [4].
in vivo
in rat brain depressing the release of hypothalamic and hypophyseal hormones, chlorprothixene blocked postsynaptic mesolimbic dopaminergic d1 and d2 receptors [5]. chlorprothixene treatment restored normal ceramide concentrations in murine bronchial epithelial cells, reduced inflammation in the lungs of mice with cystic fibrosis (cf) and prevented infection with pseudomonas aeruginosa [6].
References
[1] von coburg y, kottke t, weizel l, et al. potential utility of histamine h 3 receptor antagonist pharmacophore in antipsychotics[j]. bioorganic & medicinal chemistry letters, 2009, 19(2): 538-542.
[2] squires r f, saederup e. clozapine and several other antipsychotic/antidepressant drugs preferentially block the same ‘core’fraction of gaba a receptors[j]. neurochemical research, 1998, 23(10): 1283-1290.
[3] roth b l, craigo s c, choudhary m s, et al. binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors[j]. journal of pharmacology and experimental therapeutics, 1994, 268(3): 1403-1410.
[4] barnard d l, day c w, bailey k, et al. is the anti-psychotic, 10-(3-(dimethylamino) propyl) phenothiazine (promazine), a potential drug with which to treat sars infections: lack of efficacy of promazine on sars-cov replication in a mouse model[j]. antiviral research, 2008, 79(2): 105-113.
[5] gey k f, pletscher a. influence of chlorpromazine and chlorprothixene on the cerebral metabolism of 5-hydroxytryptamine, norepinephrine and dopamine[j]. journal of pharmacology and experimental therapeutics, 1961, 133(1): 18-24.
[6] becker k a, riethmuller j, luth a, et al. acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis[j]. american journal of respiratory cell and molecular biology, 2010, 42(6): 716-724.]
Properties of Chlorprothixene hydrochloride
| Melting point: | 224-226?C |
| storage temp. | 2-8°C |
| solubility | Soluble in water and in alcohol, slightly soluble in methylene chloride. |
| form | neat |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 6469-93-8 |
Safety information for Chlorprothixene hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Chlorprothixene hydrochloride
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran
You may like
-
 6469-93-8 Chlorprothixene hydrochloride 98%View Details
6469-93-8 Chlorprothixene hydrochloride 98%View Details
6469-93-8 -
 6469-93-8 98%View Details
6469-93-8 98%View Details
6469-93-8 -
 Chlorprothixene HCl 98.00% CAS 6469-93-8View Details
Chlorprothixene HCl 98.00% CAS 6469-93-8View Details
6469-93-8 -
 Chlorprothixene hydrochloride CAS 6469-93-8View Details
Chlorprothixene hydrochloride CAS 6469-93-8View Details
6469-93-8 -
 Chlorprothixene hydrochloride CAS 6469-93-8View Details
Chlorprothixene hydrochloride CAS 6469-93-8View Details
6469-93-8 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8
