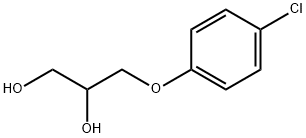
Chlorphenesin carbamate
- CAS NO.:886-74-8
- Empirical Formula: C10H12ClNO4
- Molecular Weight: 245.66
- MDL number: MFCD00072002
- EINECS: 212-954-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13

What is Chlorphenesin carbamate?
Originator
Maolate ,Upjohn ,US ,1967
The Uses of Chlorphenesin carbamate
'Muscle relaxant
The Uses of Chlorphenesin carbamate
Maolate is a pharmaceutical compound comprising naproxen.
Definition
ChEBI: The carbamate ester of the primary hydroxy group of chlorphenesin. A centrally acting skeletal muscle relaxant, it is used in the symptomatic treatment of painful muscle spasm.
Manufacturing Process
1.0 mol of 3-p-chlorophenoxy-1,2-propanediol (chlorphenesin) is suspended in
1,000 ml of benzene in a 5-liter flask equipped with a dropping funnel,
thermometer and stirrer. 1.0 mol of phosgene in 500 ml of cold, dry benzene
is then added dropwise over a period of 45 minutes, the resulting mixture
being maintained at 30°C until all solid material is dissolved. 1.0 mol of
triethylamine is added dropwise and the resulting reaction mixture stirred for
45 minutes at 30°C following the addition. The reaction mixture is then cooled
to 5°C and extracted repeatedly with 600 ml portions of cold water to remove
the triethylamine hydrochloride.
The benzene fraction, containing the intermediate 3-p-chlorophenoxy-3-
hydroxypropyl chlorocarbonate, is added to 600 ml of cold concentrated
ammonium hydroxide and the resulting reaction mixture agitated vigorously at
5°C for 7 hours. The crude 3-p-chlorophenoxy-2-hydroxypropylcarbamate
solid is then filtered off, dissolved in hot benzene, dried to remove all traces of
water, and permitted to crystallize out. Several recrystallirations from solvent
mixtures of benzene and toluene, with small amounts of acetone, produced a
crystalline white solid in about 65% yield. The product is 3-p-chlorophenoxy-
2-hydroxypropyl carbamate, melting at 89° to 91°C. The chlorphenesin
starting material is made by reacting p-chlorophenol with glyceryl
monochlorohydrin as noted in US Patent 3,214,336.
brand name
Maolate (Pharmacia & Upjohn).
Therapeutic Function
Muscle relaxant
General Description
Chlorphenesin carbamate,3-(p-chlorophenoxy)-1,2-propanediol 1-carbamate (Maolate),is the p-chloro substituted and 1-carbamate derivativeof the lead compound in the development of this group ofagents, mephenesin. Mephenesin is weakly active and shortlivedbecause of facile metabolism of the primary hydroxylgroup. Carbamylation of this group increases activity. p-Chlorination increases the lipophilicity and seals off thepara position from hydroxylation. Metabolism, still fairlyrapid, involves glucuronidation of the secondary hydroxylgroup. The biological half-life in humans is 3.5 hours.
Metabolism
Not Available
Properties of Chlorphenesin carbamate
| Melting point: | 89-91° |
| Density | 1.3336 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | -20°C, Inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 886-74-8(CAS DataBase Reference) |
Safety information for Chlorphenesin carbamate
Computed Descriptors for Chlorphenesin carbamate
Abamectin manufacturer
Synthokem Labs Private Limited
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 886-74-8 Chlorphenesin Carbamate 99%View Details
886-74-8 Chlorphenesin Carbamate 99%View Details
886-74-8 -
 Chlorphenesin carbamate 99%View Details
Chlorphenesin carbamate 99%View Details -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4