CHLOROPROCAINE
- CAS NO.:133-16-4
- Empirical Formula: C13H19ClN2O2
- Molecular Weight: 270.76
- MDL number: MFCD00864416
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
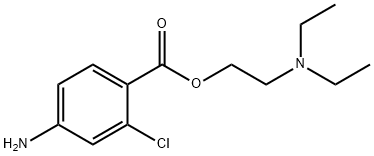
What is CHLOROPROCAINE?
Absorption
Thanks to its low risk for systemic toxicity, chloroprocaine has a rapid onset of action that usually ranges between 6 to 12 minutes. The duration of chloroprocaine-induced anesthesia may be up to 60 minutes. The absorption rate of local anesthetics depends on the total dose and concentration of chloroprocaine, as well as the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anesthetic injection. The presence of epinephrine reduces the rate of absorption and plasma concentration of local anesthetics. The systemic exposure to chloroprocaine following its topical ocular administration has not been evaluated.
Toxicity
Most chloroprocaine overdose cases are related to high plasma levels during its therapeutic use or to the unintended administration of a subarachnoid injection. In mice, the intravenous LD50 of chloroprocaine HCl is 97 mg/kg and the subcutaneous LD50 of chloroprocaine HCl is 950 mg/kg. The first consideration in the management of local anesthetic emergencies is prevention, which can be achieved by carefully monitoring patients’ cardiovascular and respiratory vital signs, as well as their state of consciousness. If there are any changes, oxygen should be administered.
In patients with chloroprocaine overdose with convulsions, underventilation or apnea, the drug label recommends giving immediate attention to maintaining a patent airway, assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. If convulsions persist, provide small increments of an ultra-short acting barbiturate or a benzodiazepine intravenously. Refer to the chloroprocaine drug label for a complete description of the procedures recommended in case of overdose.
Description
Chloroprocaine (N,N′-diethylaminoethyl 4-amino-2-chlorobenzoate) is a very short-acting, amino ester-type local anesthetic used to provide regional anesthesia by infiltration as well as by peripheral and central nerve block, including lumbar and caudal epidural blocks. The presence of a chlorine atom ortho to the carbonyl of the ester function increases its rate of hydrolysis by plasma cholinesterase at least threefold compared to procaine and benzocaine. Thus, chloroprocaine may be used in maternal and neonatal patients with minimal placental passage of chloroprocaine. The lower plasma cholinesterase activity in the maternal epidural space must still have sufficient activity for degrading chloroprocaine and, thus, not allowing it to cross the placenta barrier. Like PABA, the hydrolysis product of chloroprocaine, 4-amino-2-chlorobenzoic acid, also inhibits the action of sulfonamides. Therefore, its use with sulfonamides should be avoided.
The Uses of CHLOROPROCAINE
Chloroprocaine is an ester that is metabolised rapidly by ester hydrolysis, so its duration of action is short and potential for cardiac toxicity relatively low. It can be used as a preservative-free solution for spinal anaesthesia for surgical procedures up to 40 min in duration.
The Uses of CHLOROPROCAINE
Anesthetic (local).
Background
Chloroprocaine is an ester local anesthetic commonly available in its salt form, chloroprocaine hydrochloride. Similar to other local anesthetics, it increases the threshold for electrical excitation in nerves by slowing the propagation of the nerve impulse and reducing the rate of rise of the action potential. The pharmacological profile of chloroprocaine is characterized by a short latency and duration, similar to the one observed with lidocaine. Chloroprocaine can be given as an injection, and is available in formulations with and without methylparaben as a preservative. Both can be given as intrathecal injections for peripheral and central nerve block, but only the preservative-free formulation can be used for lumbar and caudal epidural blocks. Topical chloroprocaine for ophthalmic use was approved by the FDA in September 2022 for ocular surface anesthesia.
Indications
Chloroprocaine for intrathecal injection is indicated for the production of subarachnoid block (spinal anesthesia) in adults. It is also indicated for the production of local anesthesia by infiltration, peripheral and central nerve block, and a preservative-free form can also be used for lumbar and caudal epidural blocks. Topical chloroprocaine for ophthalmic use is indicated for ocular surface anesthesia.
Definition
ChEBI: Procaine in which one of the hydrogens ortho- to the carboxylic acid group is substituted by chlorine. It is used as its monohydrochloride salt as a local anaesthetic, particularly for oral surgery. It has the advantage over lidocaine of const icting blood vessels, so reducing bleeding.
brand name
Nesacaine (Abraxis).
Biological Functions
Chloroprocaine hydrochloride (Nesacaine) is obtained from addition of a chlorine atom to procaine, which results in a compound of greater potency and less toxicity than procaine itself. This local anesthetic is hydrolyzed very rapidly by cholinesterase and therefore has a short plasma half-life. Because it is broken down rapidly, chloroprocaine is commonly used in obstetrics. It is believed that the small amount that might get to the fetus continues to be rapidly hydrolyzed, so there may be no residual effects on the neonate.
General Description
The 2 chloride substitution on the aromatic ring of chloroprocaineis an electron-withdrawing functional group. Thus, itpulls the electron density from the carbonyl carbon into thering. The carbonyl carbon is now a stronger electrophile andmore susceptible to ester hydrolysis. Therefore, chloroprocainehas a more rapid metabolism than procaine. The in vitroplasma half-life is approximately 25 seconds. The 2-chloro-4-aminobenzoic acid metabolite precludes this from being usedin patients allergic to PABA. The very short duration of actionmeans that this drug can be used in large doses for conductionblock (with rapid onset and short duration of action.).
Pharmacokinetics
Chloroprocaine is an ester local anesthetic agent indicated for the production of local or regional anesthesia with effects on the cardiovascular and central nervous systems. Compared with lidocaine and bupivacaine, chloroprocaine has shorter ambulation and discharge times. Chloroprocaine has minimal effects on cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance at therapeutic doses. However, at toxic blood concentrations, chloroprocaine may cause atrioventricular block and cardiac arrest, as well as decreased cardiac output and arterial blood pressure. A high concentration of chloroprocaine in plasma can also lead to central nervous system stimulation, depression, or both. Some signs of central stimulation include restlessness, tremors and shivering, which may progress to convulsions. Depression, coma and respiratory arrest may also occur. As with other local anesthetics, patients may experience depression of the central nervous system without a prior stage of stimulation.
Clinical Use
Chloroprocaine is used for cutaneous or mucous membraneinfiltration for surgical procedures, epidural anesthesia(without preservatives) and for peripheral conduction block.
Metabolism
In plasma, chloroprocaine is quickly metabolized by pseudocholinesterases, a group of enzymes that perform the hydrolysis of the ester linkage. In ocular tissues, chloroprocaine is metabolized by nonspecific esterases. The hydrolysis of chloroprocaine leads to the production of ?-diethylaminoethanol and 2-chloro-4-aminobenzoic acid, which inhibits the action of the sulfonamides.
Properties of CHLOROPROCAINE
| Melting point: | 42°C |
| Boiling point: | 402.6±35.0 °C(Predicted) |
| Density | 1.2020 (rough estimate) |
| refractive index | 1.5270 (estimate) |
| pka | 9.14±0.25(Predicted) |
Safety information for CHLOROPROCAINE
Computed Descriptors for CHLOROPROCAINE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
