CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Odor | ODORLESS |
| Melting Point | 173-174ºC |
| Solubility | 0.665 mg/mL |
| LogP | 2.86 |
| Stability/Shelf Life | STABLE IN AIR /HYDROCHLORIDE/ |
| Decomposition | When heated to decomposition it emits toxic fumes of nitroxides and /hydrogen chloride/. |
| Dissociation Constants | 9 |
| Kovats Retention Index | 2229 2229 |
| Other Experimental Properties | Crystals; mp: 176-178 °C (microstage); bitter taste; aq soln are just acid to litmus and turn yellow on standing; about 1 g sol in: 22 ml water @ 20 °C, 100 ml 95% ethanol /Hydrochloride/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 270.75 g/mol |
|---|---|
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 270.1135055 g/mol |
| Monoisotopic Mass | 270.1135055 g/mol |
| Topological Polar Surface Area | 55.6 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 259 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
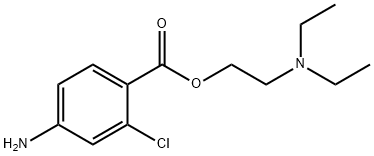
Chloroprocaine is procaine in which one of the hydrogens ortho- to the carboxylic acid group is substituted by chlorine. It is used as its monohydrochloride salt as a local anaesthetic, particularly for oral surgery. It has the advantage over lidocaine of constricting blood vessels, so reducing bleeding. It has a role as a local anaesthetic, a peripheral nervous system drug and a central nervous system depressant. It is a benzoate ester and a member of monochlorobenzenes. It is functionally related to a 2-diethylaminoethanol and a 4-amino-2-chlorobenzoic acid.