Chlorophyll A
Synonym(s):Chlorophyll a
- CAS NO.:479-61-8
- Empirical Formula: C55H72MgN4O5
- Molecular Weight: 893.49
- MDL number: MFCD00079050
- EINECS: 207-536-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-24 13:07:15
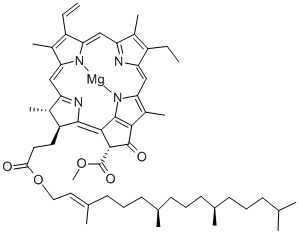
What is Chlorophyll A?
Description
Chlorophyll is the green pigment in plants that allows them to create energy from light – to photosynthesize. Chlorophyll A and B are two major types of chlorophyll found in plants and green algae. Both are involved in the process photosynthesis. Both chlorophyll A and B are found in chloroplasts, associated with integral membrane proteins in the thylakoid membrane. The main difference between chlorophyll A and B is their role in photosynthesis? chlorophyll A is the principal pigment involved in the photosynthesis whereas chlorophyll B is the accessory pigment, collecting the energy in order to pass into chlorophyll A. In chlorophyll A, the most effectively absorbing wavelengths of the spectrum are 429 nm and 659 nm, which are responsible for violet-blue and orange-red colors, respectively.
Description
Chlorophyll a is a green pigment used by plants in photosynthesis. It is also used to color some soaps, oils, and confectionaries green.
Description
Chlorophyll is the green pigment in plants, algae, and cyanobacteria that is essential for photosynthesis. Its central structure is an aromatic porphyrin or chlorin (reduced porphyrin) ring system with a sequestered magnesium atom. A fifth ring is fused to the porphyrin.
Chlorophyll is not a single molecule: There are at least six varieties that have various side groups on the rings. In most chlorophylls, one of the groups is a long phytyl ester chain.
Chlorophyll a, shown here, is called the “universal” chlorophyll because it is present in almost all photosynthetic organisms. It has been known since 1817, but scientists did not realize that it contains magnesium until 1906.
By far the most important “application” of chlorophyll is photosynthesis; but it has also been used as a green coloring agent in foods, cosmetics, soaps, and alcoholic beverages. Its ester side chain can be cleaved to obtain phytol, an alcohol used in the synthesis of vitamins E and K1. It’s even been tried as an antiknock additive for gasoline.
Why is chlorophyll green? The bonding in many metal–organic coordination compounds causes them to absorb some wavelengths of white light while reflecting others. In the case of chlorophyll, light wavelengths in the blue and red regions of the spectrum are required for the pigment to do its business. Chlorophyll absorbs them; but it does not need to use green light, which is reflected to produce the intense green color of leaves.
January 26 is National Green Juice Day. (Yes, there is such a thing.) It’s a perfect time to get your fill of chlorophyll.
Chemical properties
Waxy solid. Olive to dark green, depending on the amount of magnesium incorporated. Slightly smelly. The melting point of chlorophyll a is 150~153°C, and the melting point of chlorophyll b is 183~185°C. It coexists with the yellow carotene and lutein in the chloroplasts of plant leaves. Sensitive to light and heat, it can be saponified and hydrolyzed into bright green chlorophyllin (salt), phytol and methanol in dilute lye solution, and dark green to green-brown pheophytin in acid solution. Insoluble in water. Soluble in ethanol, ether, acetone and other fatty solvents.
Occurrence
Chlorophyll A is found in all plants, algae and cyanobacteria.
The Uses of Chlorophyll A
Chlorophyll A is a photosynthetic pigment that is essential for photosynthesis of eukaryotes and cyanobacteria, acting as a primary donor in the electron transport chain. Dyes and metabolites.
What are the applications of Application
Chlorophyll a is a form of the light absorbing pigment of algae and plants
Definition
Chlorophyll A is a form of chlorophyll that absorbs light in the violet to red spectrum (approximately 400-700 nm wavelength range) and reflects green light (500-570 nm wavelength), which imparts the characteristic green color to land plants. It is occurs in all photosynthetic organisms and is the major species in all of those that evolve oxygen.
General Description
Chlorophyll a is present as a key pigment in organisms with oxygenic photosynthesis. It functions as an antenna pigment in both reaction centers I and II.
Biochem/physiol Actions
Chlorophyll a is an essential pigment in oxygenic photosynthesis. In photosystems, it is present as an antenna pigment as well as in reaction center I and II. Chlorophyll a is a blue-green pigment which absorbs light in 400-450nm and 640-660nm range. In photosynthesis, the energy absorbed by chlorophyll transforms carbon dioxide and water into carbohydrates and oxygen. When chlorophyll absorbs light energy, an electron in chlorophyll is excited from a lower energy state to a higher energy state. In this higher energy state, the electron is more readily transferred to another molecule. This starts a chain of electron-transfer steps, which ends with an electron transferred to carbon dioxide.
Purification Methods
It forms green crystals from Me2CO, Et2O/H2O, Et2O/hexane/H2O or Et2O/pentane /H2O. It is sparingly soluble in MeOH and insoluble in pet ether. In alkaline solution it gives a blue-green colour with deep red fluorescence. A very crude chlorophyll mixture has been purified by chromatography on low melting polyethylene (MI 0.044; “Dow” melting index MI <2) and developed with 70% aqueous Me2CO. The order of effluent from the bottom of the column is: xanthophylls, chlorophyll b , chlorophyll a, phaeophytins and carotenes. A mixture of chlorophylls a and b is best separated by chromatography on sugar, and the order is chlorophyll b elutes first followed by chlorophyll a. To an Me2CO/H2O solution of chlorophylls 200mL of iso-octane are added, and the mixture shaken in a separating funnel and the H2O is carefully removed. The iso-octane layer is dried (Na2SO4) and applied onto a glass column (5cm diameter) dry packed with 1000mL of powdered sucrose which has been washed with 250mL of iso-octane. Elution with 0.5% of isopropanol in iso-octane gives chlorophyll a. Keeping the eluate overnight at 0o yields micro crystals which are collected by filtration or centrifugation (Yield 40mg). UVEtOH has max 660, 613, 577, 531, 498, 429 and 409 nm. Note that anhydrous chlorophylls can be easily enolized, epimerized or allomerized in dry polar organic solvents. [Anderson & Calvin Nature 194 285 1962, Stoll & Weidemann Helv Chim Acta 16 739 757 1933, NMR: Katz et al. J Am Chem Soc 90 6841 1968, 85 3809 1963 for a and b; ORD: Inhoffen et al. Justus Liebigs Ann Chem 704 208 1967, Willst.tter & Isler Justus Liebigs Ann Chem 390 269, 233 1912, Beilstein 26 III/IV 3243.]
Properties of Chlorophyll A
| Melting point: | 117-120° |
| alpha | D20 -262° (acetone) |
| storage temp. | -20°C |
| solubility | diethyl ether: soluble0.1mg/10 mL |
| form | powder |
| appearance | green solid |
| color | Green to Black Semi-Solid |
| Merck | 13,2174 |
| BRN | 3586237 |
| Stability: | Stable, but may discolour upon exposure to light. Incompatible with strong oxidizing agents. May be air sensitive. |
| EPA Substance Registry System | Chlorophyll a (479-61-8) |
Safety information for Chlorophyll A
Computed Descriptors for Chlorophyll A
| InChIKey | ATNHDLDRLWWWCB-AENOIHSZSA-M |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Chlorophyll A CAS 479-61-8View Details
Chlorophyll A CAS 479-61-8View Details
479-61-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
