Chloromethyl ethyl ether
- CAS NO.:3188-13-4
- Empirical Formula: C3H7ClO
- Molecular Weight: 94.54
- MDL number: MFCD00000887
- EINECS: 221-687-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
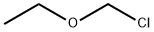
What is Chloromethyl ethyl ether?
Chemical properties
clear colorless to pale yellow liquid
The Uses of Chloromethyl ethyl ether
Chloromethyl ethyl ether can be used to prepare:
- 6-Benzyl-1-(ethoxymethyl)-5-iodopyrimidine-2,4(1H,3H)-dione, a key intermediate in the preparation of MKC-442 analog.
- Acylation catalyst for alcohols named 1,3-bis-[(R)-1-(2-naphthyl)ethyl]imidazoliumchloride by reacting with glyoxal-bis-[(R)-1-(2-naphthyl)ethyl]imine.
What are the applications of Application
Chloromethyl ethyl ether is used in the synthesis of HIV-1 reverse transcriptase inhibitiors
General Description
A colorless liquid. Slightly denser than water and insoluble in water. Flash point below 73°F. May be moderately toxic by ingestion, inhalation or skin absorption. Severely irritates skin, eyes and mucous membranes.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Chloromethyl ethyl ether tends to form unstable peroxides when exposed to oxygen or air. These products can sometimes be observed as clear crystals deposited on containers or along the surface of the liquid. Forms salts with strong acids and addition complexes with Lewis acids. May react violently with strong oxidizing agents. I
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Properties of Chloromethyl ethyl ether
| Melting point: | -104°C |
| Boiling point: | 82 °C(lit.) |
| Density | 1.019 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 67 °F |
| storage temp. | 0-6°C |
| solubility | Chloroform (Soluble), Ethyl Acetate (Slightly) |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Specific Gravity | 1.019 |
| InChI | InChI=1S/C3H7ClO/c1-2-5-3-4/h2-3H2,1H3 |
| CAS DataBase Reference | 3188-13-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethane, (chloromethoxy)-(3188-13-4) |
| EPA Substance Registry System | Ethane, (chloromethoxy)- (3188-13-4) |
Safety information for Chloromethyl ethyl ether
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Chloromethyl ethyl ether
| InChIKey | FCYRSDMGOLYDHL-UHFFFAOYSA-N |
| SMILES | CCOCCl |
Chloromethyl ethyl ether manufacturer
RVR Labs Pvt Ltd
GK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 chloromethyl ethyl ether 98%View Details
chloromethyl ethyl ether 98%View Details -
 Chloro Methyl Ethyl Ether 98%View Details
Chloro Methyl Ethyl Ether 98%View Details -
 Chloromethyl Ethyl Ether CAS 3188-13-4View Details
Chloromethyl Ethyl Ether CAS 3188-13-4View Details
3188-13-4 -
 Chloromethyl ethyl ether CAS 3188-13-4View Details
Chloromethyl ethyl ether CAS 3188-13-4View Details
3188-13-4 -
 Chloromethyl Ethyl Ether(EOM Chloride) 97%View Details
Chloromethyl Ethyl Ether(EOM Chloride) 97%View Details
3188-13-4 -
 3188-13-4 98%View Details
3188-13-4 98%View Details
3188-13-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
