Chloromethyl chloroformate
- CAS NO.:22128-62-7
- Empirical Formula: C2H2Cl2O2
- Molecular Weight: 128.94
- MDL number: MFCD00077688
- EINECS: 244-793-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 17:06:39
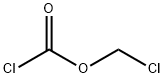
What is Chloromethyl chloroformate?
Chemical properties
Chloromethyl chloroformate is Clear Colourless Oil
The Uses of Chloromethyl chloroformate
Chloromethyl chloroformate is used as an intermediate in pharmaceuticals and in manufacture other chemicals. It acts as a reactant in the synthesis of aminocarbonyloxymethyl esters of diclofenac, flufenamic acid and in monomethoxypoly(ethyleneglycol) prodrugs of cyclosporin A.
The Uses of Chloromethyl chloroformate
Chloromethyl chloroformate is used as a reagent in the synthesis of carbamates and carbonates.
Synthesis Reference(s)
Journal of the American Chemical Society, 106, p. 1808, 1984 DOI: 10.1021/ja00318a042
General Description
Chloromethyl chloroformate is a colorless liquid with a penetrating, irritating odor. Denser than water. Contact may irritate skin, eyes and mucous membranes. Toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Air & Water Reactions
Reacts with moisture in air to generate heat and hydrochloric acid fumes [Merck, 11th ed., 1989]. Reacts with water.
Reactivity Profile
CHLOROMETHYLCHLOROFORMATE is water reactive. Incompatible with strong oxidizing agents, alcohols, bases (including amines). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Properties of Chloromethyl chloroformate
| Melting point: | <-20°C |
| Boiling point: | 107-108 °C(lit.) |
| Density | 1.449 g/mL at 20 °C |
| refractive index | n |
| Flash point: | 95°C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Soluble), Methanol (Slightly) |
| form | clear liquid |
| color | Colorless to Light yellow to Light orange |
| Water Solubility | Slightly miscible with water. |
| Sensitive | Moisture Sensitive |
| BRN | 506426 |
| Stability: | Extremely Moisture Sensitive, Hygroscopic |
| CAS DataBase Reference | 22128-62-7(CAS DataBase Reference) |
| EPA Substance Registry System | Carbonochloridic acid, chloromethyl ester (22128-62-7) |
Safety information for Chloromethyl chloroformate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chloromethyl chloroformate
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
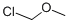


You may like
-
 Chloromethyl chloroformate CAS 22128-62-7View Details
Chloromethyl chloroformate CAS 22128-62-7View Details
22128-62-7 -
 Chloromethyl chloroformate CAS 22128-62-7View Details
Chloromethyl chloroformate CAS 22128-62-7View Details
22128-62-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4