Chlorodimethylsilane
Synonym(s):Dimethylchlorosilane;DMCS
- CAS NO.:1066-35-9
- Empirical Formula: C2H7ClSi
- Molecular Weight: 94.62
- MDL number: MFCD00000495
- EINECS: 213-912-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-09 08:40:24
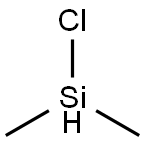
What is Chlorodimethylsilane?
Chemical properties
Colorless clear liquid
The Uses of Chlorodimethylsilane
Chlorodimethylsilane is used to prepare vinyldimethylsilanols for palladium-catalyzed cross-coupling with aryl iodides. Chlorodimethylsilane in combination with a tertiary amine such as triethylamine is another useful reagent for the synthesis of hydrodimethylsilyl ethers, especially for large scale preparations.
What are the applications of Application
The popular uses of chlorodimethylsilane are listed as below:
Will form high-boiling polymeric by-products with aqueous work-up.
A monomer ('building block') in the production of silicone polymers. Silicone polymers may be oils, greases, rubbers and resins and have a wide-range of uses.
In the electronics industry for the production of ultra-pure polysilicon and in the manufacture of semiconductors and photovoltaics.
Although the end uses of products made from chlorodimethylsilane will vary, it can be assumed that due to its highly reactive nature, no residual unreacted material will be present in any of the final products.
General Description
A colorless fuming liquid with a pungent, burning odor. Flash point -18°F. Boiling point 95°F. Density about 6.9 lb / gal. Extremely corrosive to skin and respiratory tissues, both as a liquid and vapor. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Reacts violently on contact with water to liberate heat and hydrogen chloride gas.
Reactivity Profile
Chlorosilanes, such as DIMETHYLCHLOROSILANE, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Extremely flammable
Properties of Chlorodimethylsilane
| Melting point: | −111 °C(lit.) |
| Boiling point: | 34.7 °C(lit.) |
| Density | 0.852 g/mL at 25 °C(lit.) |
| vapor density | 4 (vs air) |
| vapor pressure | 8.56 psi ( 20 °C) |
| refractive index | n |
| Flash point: | −20 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with organic solvents. |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.868 |
| explosive limit | 3.0-20.0%(V) |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1633434 |
| CAS DataBase Reference | 1066-35-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, chlorodimethyl-(1066-35-9) |
| EPA Substance Registry System | Silane, chlorodimethyl- (1066-35-9) |
Safety information for Chlorodimethylsilane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H224:Flammable liquids H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chlorodimethylsilane
| InChIKey | YGHUUVGIRWMJGE-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

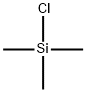

You may like
-
 Chlorodimethylsilane CAS 1066-35-9View Details
Chlorodimethylsilane CAS 1066-35-9View Details
1066-35-9 -
 Chlorodimethylsilane CAS 1066-35-9View Details
Chlorodimethylsilane CAS 1066-35-9View Details
1066-35-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
