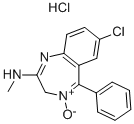
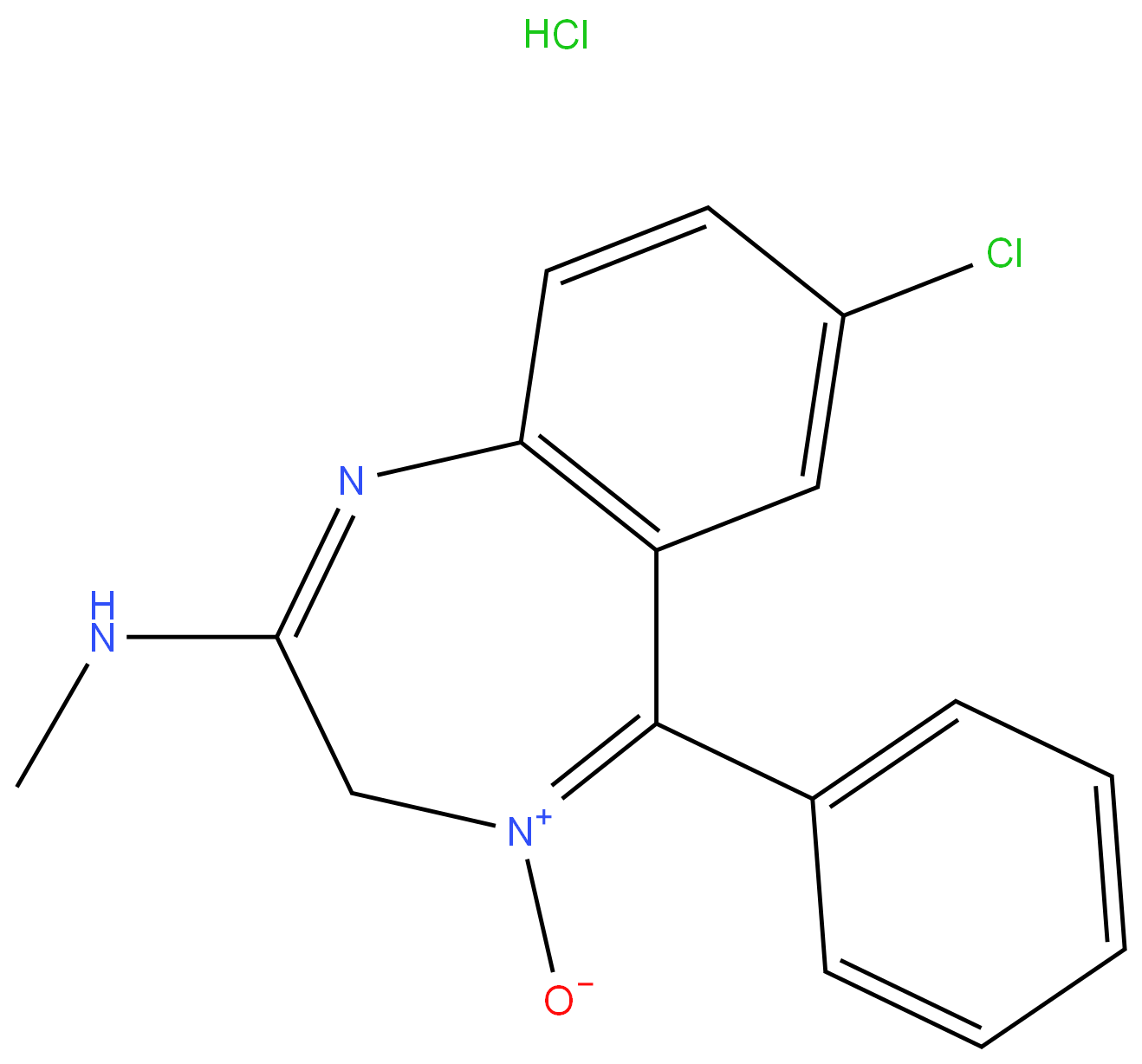
CHLORDIAZEPOXIDE HYDROCHLORIDE
Synonym(s):7-Chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine 4-oxide hydrochloride
- CAS NO.:438-41-5
- Empirical Formula: C16H15Cl2N3O
- Molecular Weight: 336.22
- MDL number: MFCD00069223
- EINECS: 207-117-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is CHLORDIAZEPOXIDE HYDROCHLORIDE?
Chemical properties
white crystalline powder
Originator
Librium,Roche,W. Germany,1960
The Uses of CHLORDIAZEPOXIDE HYDROCHLORIDE
Sedative-hypnotic.
Manufacturing Process
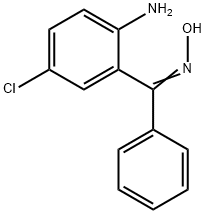
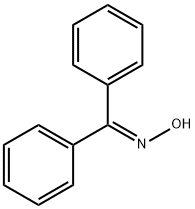
A mixture of 202 g 2-amino-5-chlorobenzophenone, 190 g hydroxylamine
hydrochloride, 500 cc pyridine and 1,200 cc alcohol was refluxed for 16 hours,
then concentrated in vacuo to dryness. The residue was treated with a
mixture of ether and water. The water was separated, the ether layer
containing a considerable amount of precipitated reaction product was washed
with some water and diluted with petroleum ether. The crystalline reaction
product, 2-amino-5-chlorobenzophenone-alpha-oxime, was filtered off. The
product was recrystallized from a mixture of ether and petroleum ether
forming colorless prisms, MP 164 to 167°C.
To a warm solution (50°C) of 172.5 g (0.7 mol) of 2-amino-5-
chlorobenzophenone-alpha-oxime in one liter glacial acetic acid were added
110 cc (1.47 mols) chloroacetyl chloride. The mixture was heated for 10
minutes at 50°C and then stirred at room temperature for 15 hours. The
precipitated yellow prisms, 2-chloromethyl-4-phenyl-6-chloroquinazoline 3-
oxide hydrochloride, were filtered off, melting range 128° to 150°C with dec.
The acetic acid mother liquor, containing the rest of the reaction product, was
concentrated in vacuo. The residue was dissolved in methylene chloride and
washed with ice cold sodium carbonate solution. The organic solution was
dried, concentrated in vacuo to a small volume and diluted with ether and
petroleum ether. Fine yellow needles of 2-chloromethyl-4-phenyl-6-
chloroquinazoline 3-oxide precipitated. The pure base was recrystallized from
a mixture of methylene chloride, ether and petroleum ether, MP 133° to
134°C.
Ninety-eight grams of 6-chloro-2-chloromethyl-4-phenylquinazoline 3-oxide
hydrochloride were introduced into 600 cc of ice cold 25% methanolic
methylamine. The mixture was initially cooled to about 30°C and then stirred
at room temperature. After 15 hours the reaction product which precipitated
was filtered off. The mother liquor was concentrated in vacuo to dryness. The
residue was dissolved in methylene chloride, washed with water and dried
with sodium sulfate. The methylene chloride solution was concentrated in
vacuo and the crystalline residue was boiled with a small amount of acetone
to dissolve the more soluble impurities. The mixture was then cooled at 5°C
for 10 hours and filtered. The crystalline product, 7-chloro-2-methylamino-5-
phenyl-3H-1,4-benzodiazepine 4-oxide, was recrystallized from ethanol
forming light yellow plates, MP 236° to 236.5°C.
A solution of 7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepine 4-oxide
in an equivalent amount of methanolic hydrochloric acid was diluted with
ether and petroleum ether.
The precipitated hydrochloride was filtered off and recrystallized from
methanol, MP 213°C.
brand name
Librium (Valeant).
Therapeutic Function
Tranquilizer
General Description
Crystals or off-white powder.
General Description
Chlordiazepoxide hydrochloride, 7-chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine4-oxide monohydrochloride (Librium), is well absorbedafter oral administration. Peak plasma levels are reachedin 2 to 4 hours. The half-life of chlordiazepoxide is 6 to30 hours. N-demethylation and hydrolysis of the condensedamidino group are rapid and extensive, producing demoxepamas a major metabolite. Demoxepam can undergo fourdifferent metabolic fates. It is converted principally to itsactive metabolite nordazepam, which is also a major activemetabolite of diazepam, clorazepate, and prazepam.Nordazepam, in turn, is converted principally to active oxazepam(marketed separately), which conjugated to the excretedglucuronide. Because of the long half-life of parentdrug and its active metabolites, this drug is long acting andself-tapering. As with diazepam (vide infra), repeated administrationof chlordiazepoxide can result in accumulationof parent drug and its active metabolites, and thus cause excessivesedation.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Acidic salt of an amine. Materials in this group are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
CNS depressant. Manufacture and dosage controlled by law.
Fire Hazard
Flash point data for CHLORDIAZEPOXIDE HYDROCHLORIDE are not available. CHLORDIAZEPOXIDE HYDROCHLORIDE is probably combustible.
Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion and subcutaneous routes. An experimental teratogen. Experimental reproductive effects. Human systemic effects: ataxia, dlstorted perceptions, hallucinations, somnolence, and surface EEG changes. Mutation data reported. A minor tranquhzer. When heated to decomposition it emits very toxic fumes of HCl and NOx.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism possibly increased by
rifampicin.
Antipsychotics: enhanced sedative effects; serious
adverse events reported with clozapine and
benzodiazepines.
Antivirals: concentration possibly increased by
ritonavir.
Sodium oxybate: enhanced effects of sodium oxybate
- avoid.
Ulcer-healing drugs: metabolism inhibited by
cimetidine.
Metabolism
Chlordiazepoxide is extensively metabolised in the liver.
The elimination half-life of chlordiazepoxide ranges
from about 6-30 hours, but its main active metabolite
desmethyldiazepam (nordazepam) has a half-life of
several days. Other pharmacologically active metabolites
of chlordiazepoxide include desmethylchlordiazepoxide,
demoxepam, and oxazepam.
Unchanged drug and metabolites are excreted in the
urine, mainly as conjugated metabolites.
Properties of CHLORDIAZEPOXIDE HYDROCHLORIDE
| Melting point: | 213° |
| solubility | Soluble in water, sparingly soluble in ethanol (96 per cent). |
| form | neat |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 438-41-5(CAS DataBase Reference) |
| EPA Substance Registry System | Chlordiazepoxide hydrochloride (438-41-5) |
Safety information for CHLORDIAZEPOXIDE HYDROCHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for CHLORDIAZEPOXIDE HYDROCHLORIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
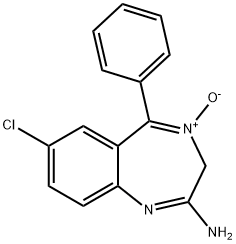
You may like
-
 438-41-5 Chlordiazepoxide hydrochloride 98%View Details
438-41-5 Chlordiazepoxide hydrochloride 98%View Details
438-41-5 -
 438-41-5 99%View Details
438-41-5 99%View Details
438-41-5 -
 Chlordiazepoxide hydrochloride 438-41-5 98%View Details
Chlordiazepoxide hydrochloride 438-41-5 98%View Details
438-41-5 -
 438-41-5 98%View Details
438-41-5 98%View Details
438-41-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
